Chemistry Ph Worksheet Answers Revealed: Boost Your Understanding

The world of chemistry, while immensely rewarding, can often feel like a labyrinth of symbols, equations, and concepts that are difficult to navigate. Whether you're a student, a hobbyist, or just someone interested in the fundamentals of science, understanding the Periodic Table is crucial. In this comprehensive guide, we will delve into one of the most fundamental tools in chemistry education - the Periodic Table, more specifically focusing on "Chemistry pH Worksheet Answers."
Understanding the Basics of the Periodic Table

Before we dive into pH worksheets, let's touch upon the basics of the Periodic Table:
- Organization: Elements are arranged by increasing atomic number, reflecting the number of protons in the nucleus.
- Periods and Groups: The horizontal rows are called periods, while the vertical columns are groups or families, with similar properties.
- Elements: From Hydrogen to Oganesson, each element has unique characteristics that can affect their pH behavior.
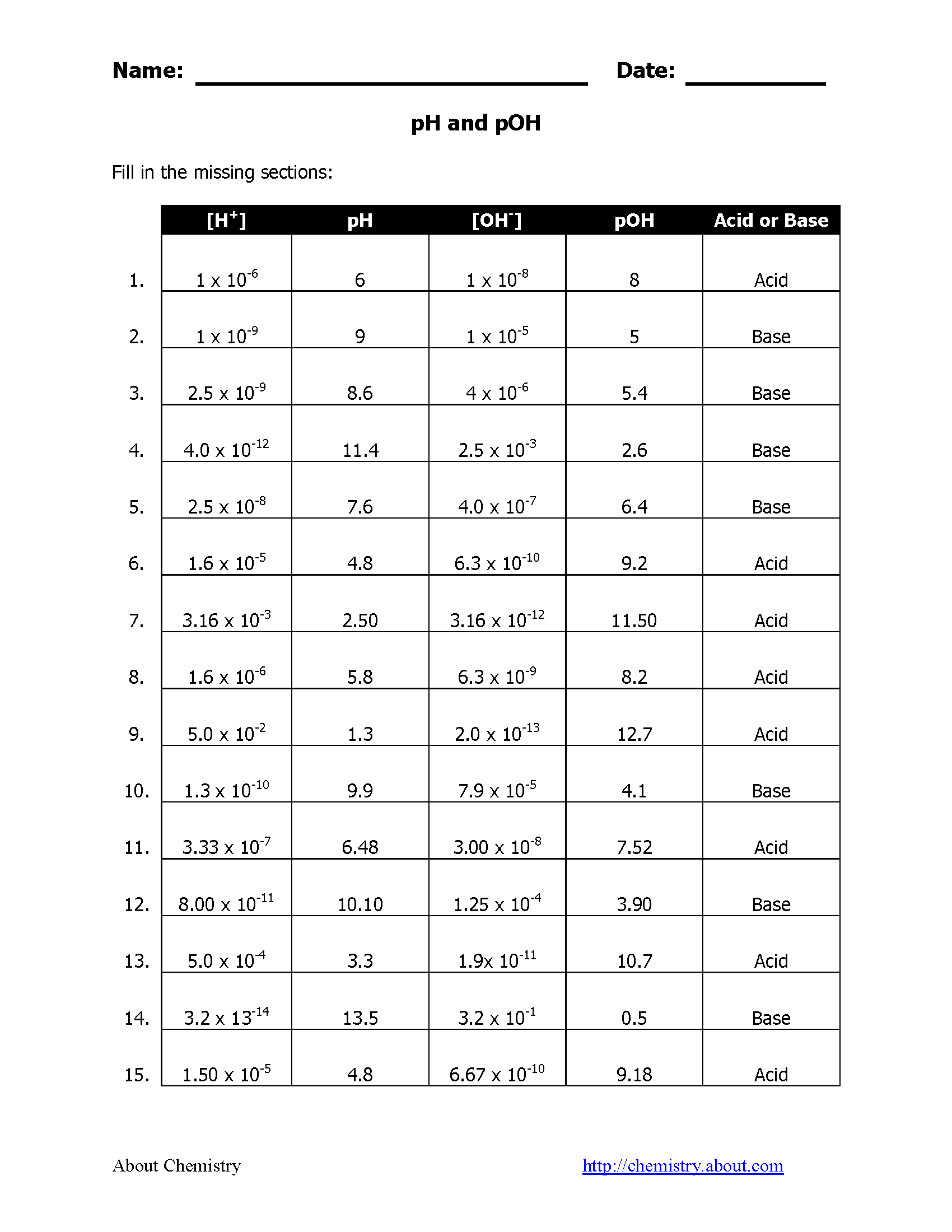
Here's a quick visual of what we're discussing:
Chemistry pH Worksheets and Their Significance

pH worksheets are designed to help students grasp the concept of pH, which measures the acidity or alkalinity of solutions. Understanding pH is essential in both theoretical and practical chemistry applications, from environmental science to industrial processes.
The worksheets usually cover:
- Calculation of pH from given ion concentrations.
- Acid-base titrations and how they relate to pH changes.
- Understanding the role of indicators in determining pH.
Step-by-Step Guide to Solving pH Worksheet Questions

Question Types and Their Answers

Let's explore some common question types found in pH worksheets:
- Calculating pH from Hydrogen Ion Concentration: Given [H+] = 1x10-3 M, you find pH using the formula pH = -log[H+]. Here, pH = -log(1x10-3) = 3.
- Calculating pH from Hydroxide Ion Concentration: If you're given [OH-] = 1x10-8 M, you first need to find [H+] using Kw = 1x10-14. Then, pH = 14 - pOH (where pOH = -log[OH-]).
- Using Indicators: pH worksheets often ask you to predict the color of an indicator at a certain pH. For example, methyl orange turns yellow in acidic solutions and red in basic solutions.
Practical Examples

Let's take a look at some practical examples:
- Example 1: Determine the pH of a 0.01 M solution of HCl. Since HCl is a strong acid, it completely dissociates, [H+] = 0.01 M, pH = 2.
- Example 2: A solution has a pOH of 4. What is the pH? pH = 14 - pOH = 10.
🧪 Note: When dealing with logarithmic scales like pH, remember that each unit change represents a tenfold change in concentration.
Conclusion

Understanding the pH of solutions is a cornerstone in the study of chemistry. By mastering how to calculate pH, interpret pH indicators, and connect pH with chemical behavior, you enhance not only your theoretical knowledge but also your practical skills in laboratories and beyond. The Periodic Table and pH worksheets serve as a bridge to make these complex concepts accessible and comprehensible. Next time you encounter a pH problem, remember the systematic approaches and formulas outlined here to unlock the secrets of the chemical world around us.
What does pH stand for?
+
pH stands for “power of hydrogen,” representing the concentration of hydrogen ions in a solution.
Why do we use logarithmic scales like pH?

+
Logarithmic scales allow for the representation of vast ranges of concentrations in a manageable scale, especially when dealing with exponentially changing values.
How do indicators work?

+
Indicators change color in response to the concentration of hydrogen ions, providing a visual cue for pH determination.
Can pH go below zero or above 14?

+
Technically, pH can go below zero and above 14 in highly acidic or basic solutions, respectively. However, these values are less common in everyday chemistry.
How does the Periodic Table relate to pH?

+
The Periodic Table helps us understand which elements can form acids or bases, how they interact, and their behavior in solutions, influencing pH values.