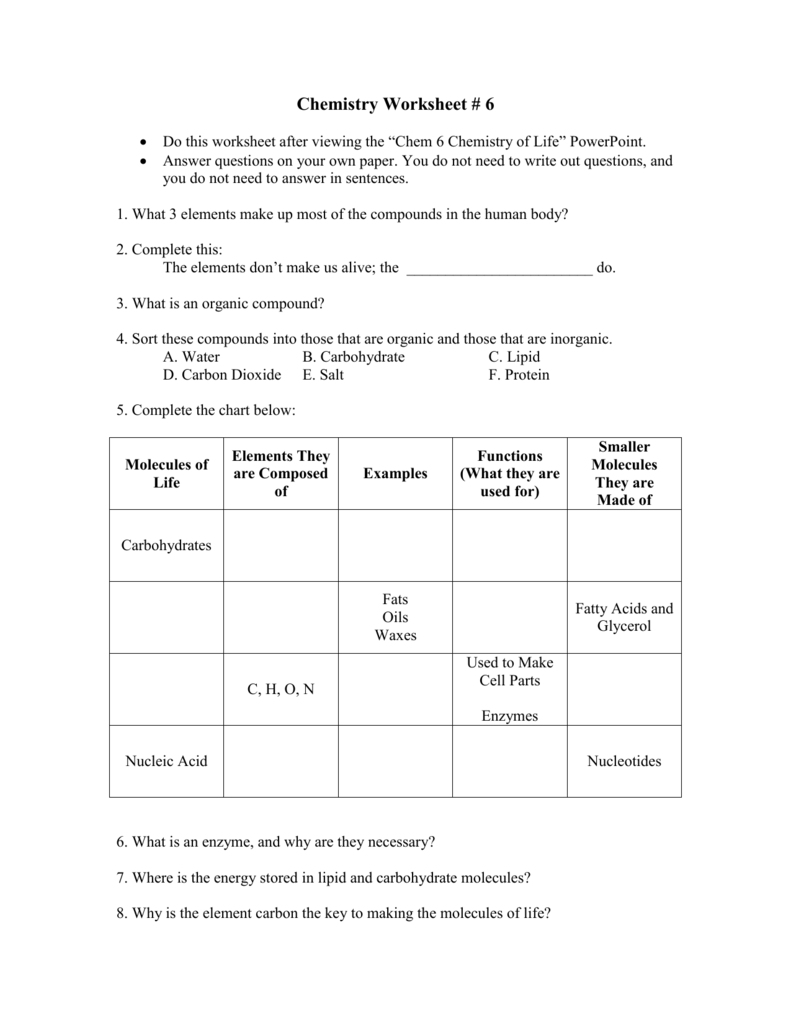
5 Essential Answers for Chemistry of Life Worksheet 1

The study of the chemistry of life, or biochemistry, is fundamental for understanding how living organisms function. This topic not only sheds light on biological processes but also aids in advancements in medicine, agriculture, and environmental sciences. In this blog post, we delve into five essential answers for a typical Chemistry of Life worksheet, providing clear explanations and examples to demystify some of the core principles of biochemistry.
What are Macromolecules?

Macromolecules are large, complex molecules that play critical roles in the structure and function of living organisms. They are typically composed of thousands of smaller units called monomers linked together to form polymers. Here are the four main classes of macromolecules:
- Proteins: Made from amino acids, proteins are involved in virtually every cell function, from catalysis to DNA replication.
- Carbohydrates: These include sugars, starches, and fibers, providing energy and structural support in cells.
- Lipids: Known for their hydrophobic properties, lipids are key components of cell membranes and energy storage.
- Nucleic Acids: DNA and RNA store and transmit genetic information, essential for life.
🌱 Note: While macromolecules are large, their sizes vary significantly. The smallest macromolecules can be a few thousand Daltons (the unit of molecular weight), while the largest, like some viruses, can reach several million Daltons.
How Do Enzymes Catalyze Reactions?

Enzymes are proteins that act as biological catalysts, accelerating chemical reactions in cells. Here’s how they do it:
- Binding: Enzymes bind to specific substrates, forming an enzyme-substrate complex.
- Transition State: The binding stabilizes the transition state, lowering the activation energy required for the reaction.
- Catalytic Site: Within the active site, various mechanisms like acid-base catalysis, covalent catalysis, or metal ion catalysis occur to speed up reactions.

What is the Role of Water in Life Processes?

Water is often called the “solvent of life” due to its unique properties:
- Polarity: Water's polar nature allows it to dissolve many substances, making it an excellent medium for chemical reactions.
- Cohesion and Adhesion: These properties help in capillary action and the transport of nutrients within plants and animals.
- Temperature Regulation: Water has a high specific heat capacity, aiding in maintaining stable internal environments.
- Chemical Reactions: Many biological reactions require or produce water.
💧 Note: The structure of water is critical; its ability to form hydrogen bonds is what gives water its unique properties essential for life.
How Do pH Levels Impact Biological Processes?

The pH level of a solution is a measure of its hydrogen ion concentration, and it significantly affects biological systems:
| pH Range | Effect on Biology |
|---|---|
| 1 - 6 | Acidic conditions can denature proteins, inhibit enzyme function, and disrupt cellular metabolism. |
| 7 | Neutral pH, the optimal range for many enzymes and biochemical reactions. |
| 8 - 14 | Basic conditions can also disrupt enzyme activity and cellular structures. |

Most biological systems require a tightly regulated pH to function correctly, with even small changes potentially leading to severe dysfunctions.
What are the Functions of Nucleotides?

Nucleotides are the building blocks of nucleic acids (DNA and RNA) and have several important roles:
- Energy Currency: ATP (Adenosine Triphosphate) acts as the primary energy transfer molecule in cells.
- Genetic Information Storage: Nucleotides form the sequences of DNA and RNA, storing genetic code.
- Enzyme Cofactors: Some nucleotides serve as coenzymes or cofactors in enzymatic reactions.
- Signaling Molecules: Nucleotides like cAMP act as secondary messengers in signal transduction pathways.
To wrap up, the chemistry of life worksheet focuses on fundamental aspects that are crucial for understanding biological processes. From macromolecules providing the building blocks for life to enzymes accelerating life-sustaining reactions, water's unique properties supporting life, pH regulation ensuring cellular function, and nucleotides managing energy and genetic information, each component is pivotal. These answers not only help in completing worksheets but also enhance our grasp on the biochemical underpinnings of life, fostering a deeper appreciation for the complexity and beauty of life at the molecular level.
What is the difference between monomers and polymers?
+
Monomers are small, individual molecules that can join together to form larger structures called polymers. For instance, amino acids are monomers that polymerize to form proteins.
Why is water considered the solvent of life?

+
Water is known as the solvent of life due to its ability to dissolve many biological molecules, its role in thermal regulation, and its participation in a wide array of biochemical reactions.
How does pH affect enzyme activity?

+
Enzyme activity is pH-dependent because each enzyme has an optimal pH range where it functions most effectively. Outside this range, the enzyme’s active site can change shape (denature), reducing or stopping activity.