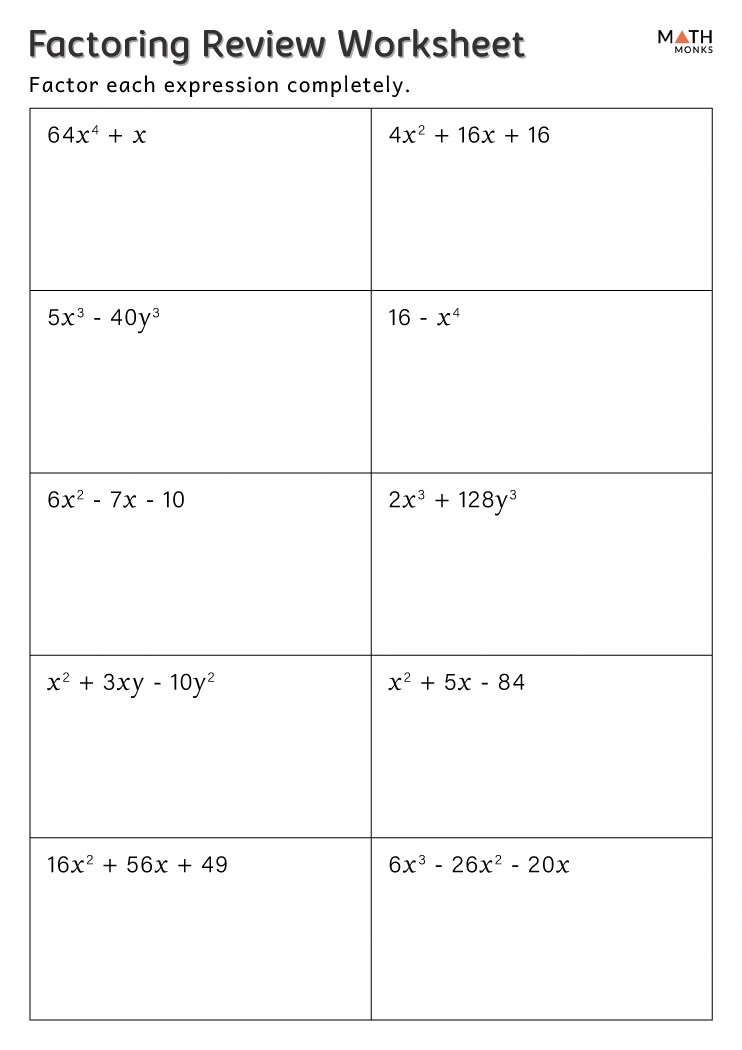
5 Key Answers for Chemistry Measurement Worksheet

Chemistry measurement worksheets are essential tools for students learning the fundamentals of chemical calculations, dimensional analysis, and unit conversions. They form the bedrock of many scientific experiments and theories, helping students develop precision, accuracy, and confidence in handling data. Here are 5 key answers to common questions you'll encounter on a chemistry measurement worksheet:
The Importance of Unit Conversions

- Understanding Units: Grasping different units of measurement, such as grams, moles, liters, and more, is crucial for effective chemistry.
- Converting Between Units: Learn how to convert quantities from one unit to another, essential for stoichiometry and reaction calculations.
- Dimensional Analysis: A powerful technique where you cancel units to solve problems; it’s particularly useful in chemistry.
Mastering unit conversions is pivotal because it not only streamlines problem-solving but also ensures accuracy in your experimental results.
Calculating Molar Mass

The molar mass, or the mass of one mole of a substance, is a fundamental concept in chemistry:
- Atomic Mass: Sum the atomic masses of all atoms present in a molecule to get the molar mass.
- Using the Periodic Table: Employ the periodic table to find the atomic masses, and ensure to consider isotopes for a more accurate calculation.
- Working with Compounds: For compounds, add up the molar masses of each element, accounting for the number of atoms per molecule.
Knowing the molar mass is essential when converting between mass and moles or when balancing chemical equations.
Determining Molecular Weight

Often confused with molar mass, molecular weight also plays a crucial role:
- Definition: Molecular weight is the mass of a single molecule, typically in atomic mass units (amu).
- Calculation: Add the mass of each atom in the molecule, not considering moles.
The distinction between molecular weight and molar mass can be confusing, but understanding this difference is key for accurate chemical analysis.
Using Avogadro’s Number

Avogadro's number (6.022 x 1023) is a constant that links the macroscopic world to the atomic scale:
- Moles and Particles: Relate moles to the number of particles in a substance using Avogadro’s number.
- Conversions: Convert from moles to molecules or atoms using this constant.
Understanding Avogadro’s number is not just about calculations; it offers a glimpse into the vastness of chemical systems at the atomic level.
Concentration Calculations

| Concentration Term | Formula |
|---|---|
| Molarity | M = n/V, where n = moles, and V = volume in liters |
| Molality | m = moles of solute/kg of solvent |
| Mole Fraction | χ = n/total number of moles |
| Parts per Million (ppm) | ppm = (solute mass/total mass) x 1,000,000 |

Concentration calculations are critical for understanding reactions in solution and the behavior of solutes:
- Importance: Concentration provides insight into the amount of a substance in a solution.
- Units: Different units like molarity, molality, and parts per million each have their applications.
Mastering these calculations allows you to prepare, dilute, and analyze solutions effectively.
🌟 Note: Practice makes perfect. The more problems you solve on chemistry measurement worksheets, the more comfortable you'll become with these concepts.
In wrapping up this guide to the essentials of chemistry measurements, it’s clear that precision and accuracy in calculations are the foundation of any successful chemical experiment or analysis. From converting units to understanding the molar mass, each of these key answers provides not just a method to solve problems but also insights into the very nature of matter and its interactions.
Why are unit conversions important in chemistry?

+
Unit conversions are crucial in chemistry because they ensure consistency in measurements and facilitate the accurate analysis of experimental data. Converting units allows for the comparison and communication of results, enabling scientists to work with different measurement systems seamlessly.
How does molar mass differ from molecular weight?

+
Molar mass is the mass of one mole of a substance in grams, while molecular weight is the mass of a single molecule in atomic mass units (amu). Molar mass is used to convert between moles and grams, whereas molecular weight is useful for theoretical calculations of individual molecules.
What does Avogadro’s number tell us?
+
Avogadro’s number (6.022 x 1023) quantifies the number of particles in one mole of any substance, bridging the gap between the microscopic world of atoms and molecules to the macroscopic scale of moles and measurable quantities.