7 Easy Tips for Balancing Chemical Equations

Balancing chemical equations is a fundamental skill in chemistry, essential for understanding chemical reactions at both an academic and professional level. However, many students and budding chemists find this task daunting. Here are seven easy tips to help you balance chemical equations efficiently and accurately:
Understand the Law of Conservation of Mass
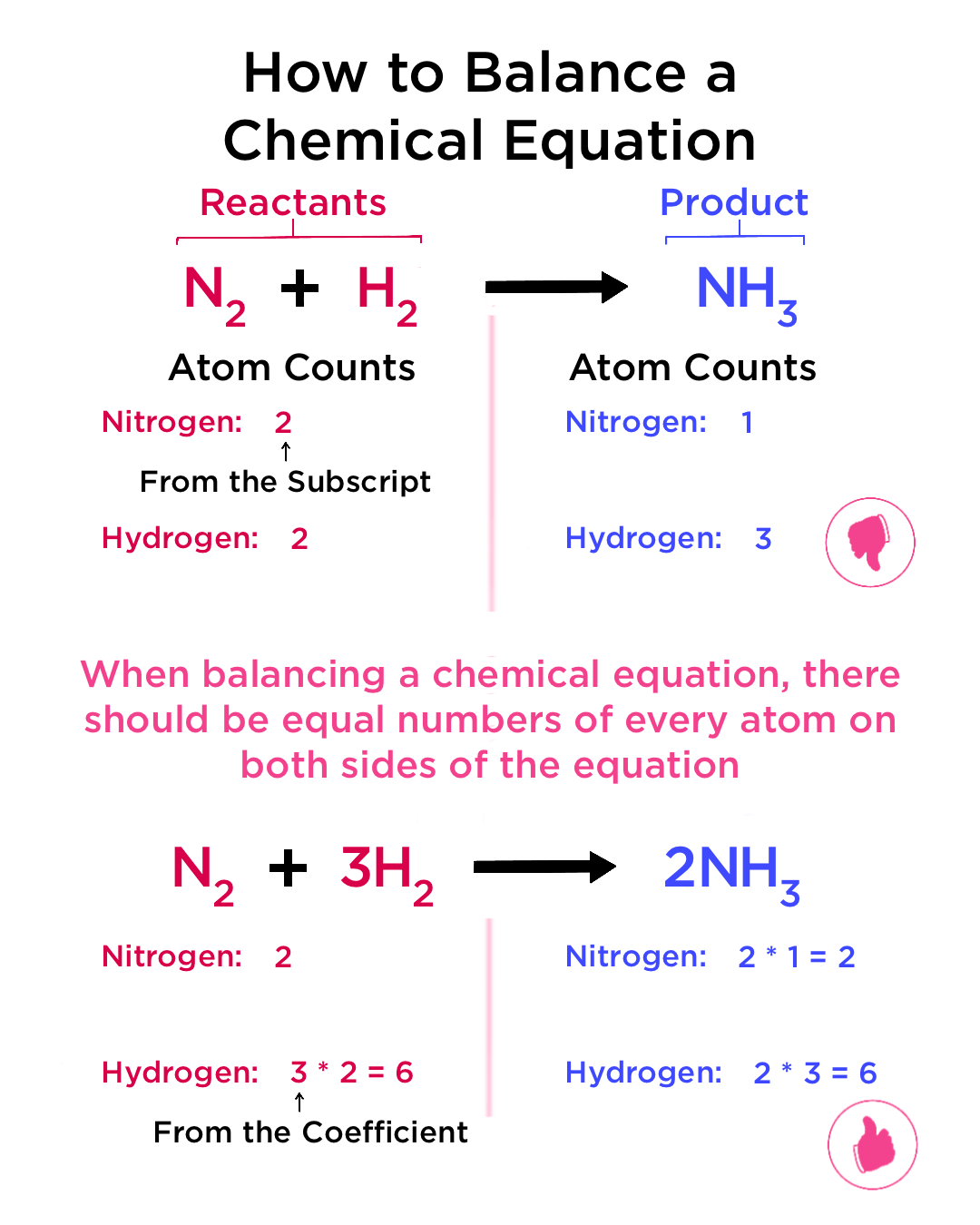
Conservation of Mass: One of the cardinal rules in chemistry is that matter cannot be created or destroyed. Thus, in a chemical reaction, the total number of atoms of each element must be the same before and after the reaction. This principle is key to mastering the art of balancing equations.
💡 Note: Before attempting to balance any equation, verify that it adheres to this law.
Start with the Most Complex Molecule

- Identify the most intricate compound in your equation. This is typically the one with the highest number of different elements.
- Balance this molecule first to establish a base for balancing the rest of the equation. This strategy often makes the task simpler by reducing the variables you need to manage simultaneously.
Balance the Elements One at a Time

Focus on one element at a time, starting with those that appear only once on each side of the equation. Here's how:
- Balance elements not part of a molecule or compound first.
- Move to polyatomic ions if present, keeping them intact to simplify balancing.
- Finally, balance elements that are part of compounds or complex molecules.
Use Coefficients Wisely

| Element | Before Balance | After Balance |
|---|---|---|
| Hydrogen | 2 | 2 |
| Oxygen | 1 | 1 |

Change the number of molecules or atoms using coefficients rather than altering the chemical formulas. Remember, only whole numbers are used for coefficients in balanced equations. Start with the smallest coefficients to keep the equation as simple as possible.
The Inspection Method

If you're new to balancing equations, begin with the inspection method:
- Identify elements that need balancing.
- Adjust coefficients by inspecting how changing one affects others, systematically moving through elements.
This method might seem time-consuming, but it ensures you understand each step's logic. Over time, you'll develop an intuitive sense of how to balance equations quickly.
Use the Half-Reaction Method for Redox Reactions

Redox reactions (where oxidation and reduction occur) often require a different approach:
- Write out the skeletal equation.
- Split it into half-reactions for oxidation and reduction.
- Balance each half-reaction separately for mass and charge.
- Recombine to form the overall balanced equation.
This method is particularly useful for reactions in an aqueous environment or in electrochemistry.
Check Your Work

After balancing:
- Count the atoms on both sides of the equation to ensure they match.
- Double-check that the total charge remains the same, especially for redox reactions.
- Verify the smallest whole-number coefficients are used.
⚙️ Note: Revisit any mistakes and refine your approach to avoid future errors.
Balancing chemical equations is not merely an academic exercise but a critical analytical skill that can be mastered through practice and understanding the underlying principles. As you apply these tips, remember that each balanced equation tells a story of chemical transformation, providing insights into how elements interact and combine to form new substances. By focusing on one step at a time and using these systematic techniques, you'll enhance your ability to balance chemical equations efficiently, making complex reactions more understandable and manageable.
Why is it important to balance chemical equations?

+
Balancing chemical equations ensures that the law of conservation of mass is satisfied. It provides a correct representation of the stoichiometry of a reaction, showing how many molecules or atoms participate in the reaction, which is crucial for understanding reaction mechanisms, predicting product quantities, and maintaining accuracy in chemical calculations.
What if I can’t balance an equation by inspection?

+
Some equations are more complex or involve redox reactions, where a method like the half-reaction approach or algebra balancing might be necessary. These methods consider changes in oxidation states or use algebraic equations to solve for coefficients, providing a more systematic approach to balancing complex equations.
How can I practice balancing equations?

+
Utilize online resources, textbook exercises, or chemical equation balancing apps. Start with simple reactions and progressively work your way to more complex ones. Writing out equations manually can also reinforce the process and improve your speed and accuracy in balancing.