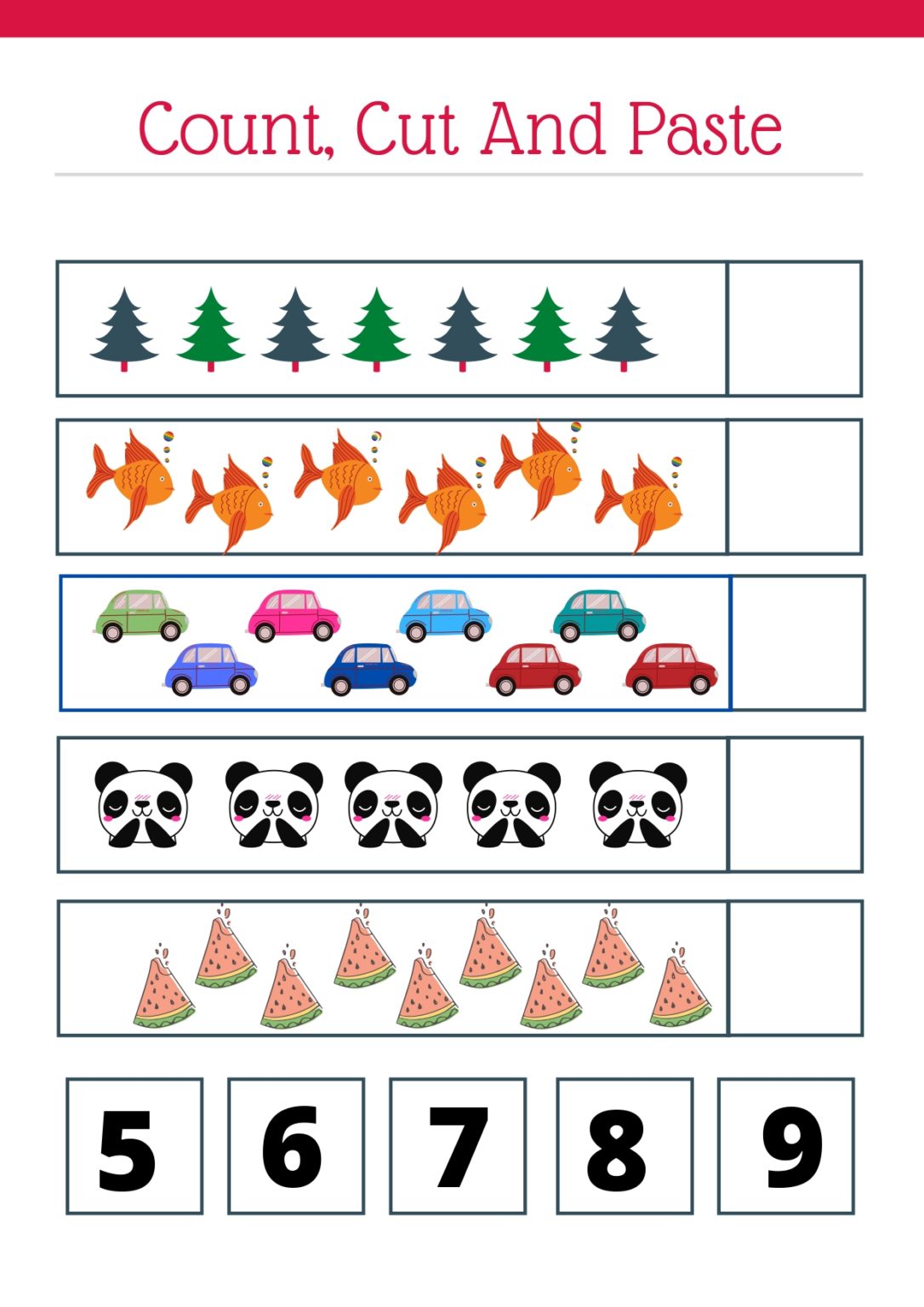
5 Essential Tips for Balancing Chemical Equations Worksheet
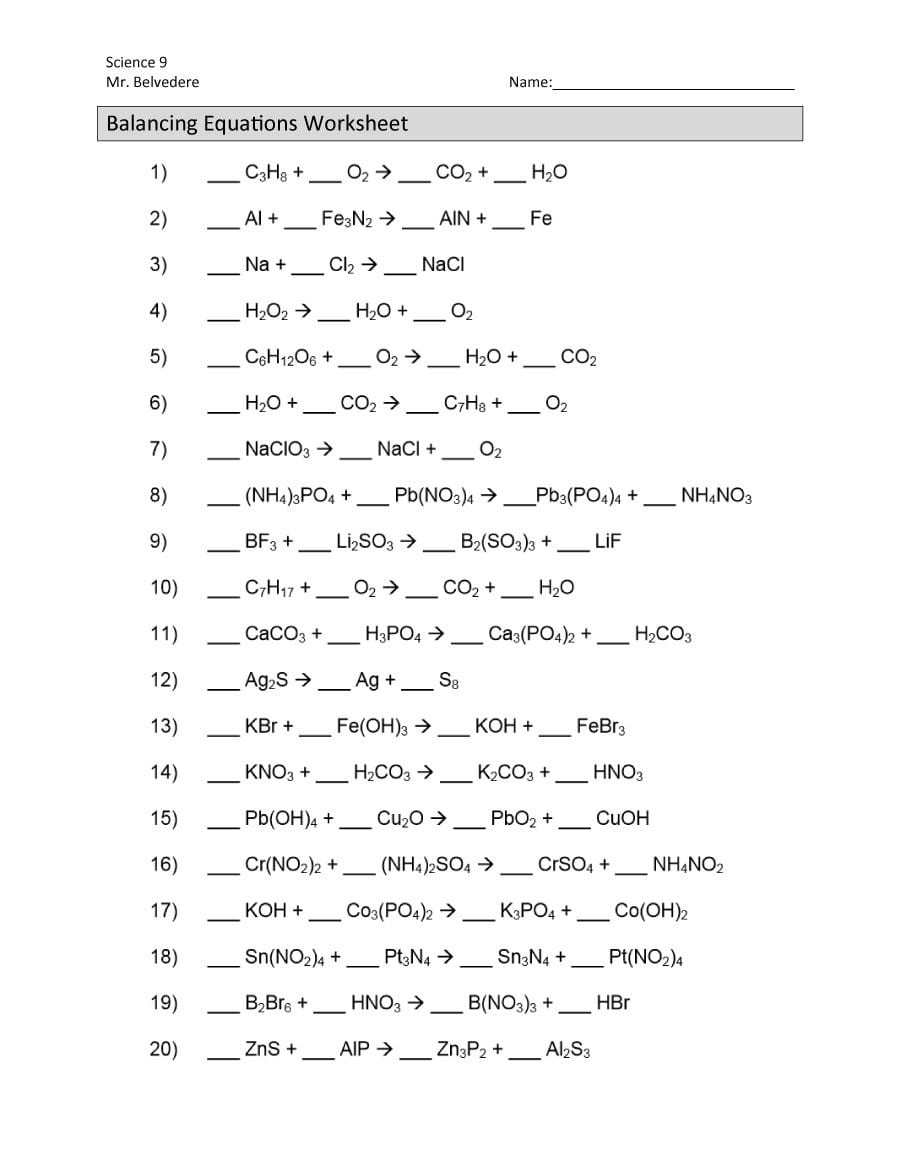
Balancing chemical equations is a fundamental skill in chemistry that ensures the law of conservation of mass is upheld in reactions. This concept, often introduced early in chemistry education, forms the bedrock upon which more complex chemical reactions are built. For students and enthusiasts looking to master this skill, there are several essential tips to keep in mind when tackling the balancing chemical equations worksheet. Let's dive into these tips to help you excel in this crucial area of chemistry.
Understanding the Basics of Chemical Equations

Before you can balance a chemical equation, you need to understand what it represents. Chemical equations provide a representation of a chemical reaction where:
- Reactants are on the left side of the equation.
- Products are on the right side.
- A single arrow (→) shows the direction of the reaction.
⚗️ Note: The symbols and formulas used must correctly represent the substances involved in the reaction.
Tip 1: Start with Simple Compounds

When you begin balancing an equation, focus first on compounds that appear only once on each side of the equation. This often includes elements or diatomic molecules like H2, O2, or N2.
- Identify which elements appear in only one reactant and one product.
- Balance these elements first to simplify subsequent balancing.
An example might look like this:
| Reactants | → | Products |
|---|---|---|
| CH4 + O2 | → | CO2 + H2O |

🔬 Note: This approach makes the balancing process more manageable.
Tip 2: Work in Steps with Oxygen and Hydrogen

Oxygen and hydrogen are usually part of many compounds in reactions. Here's how you can handle them:
- Balance oxygen after other elements because it often appears in multiple places.
- Hydrogen can sometimes be balanced last or before oxygen.
⚠️ Note: Hydrogen and oxygen should be your last considerations due to their versatility in bonding.
Tip 3: Use Fractions When Necessary
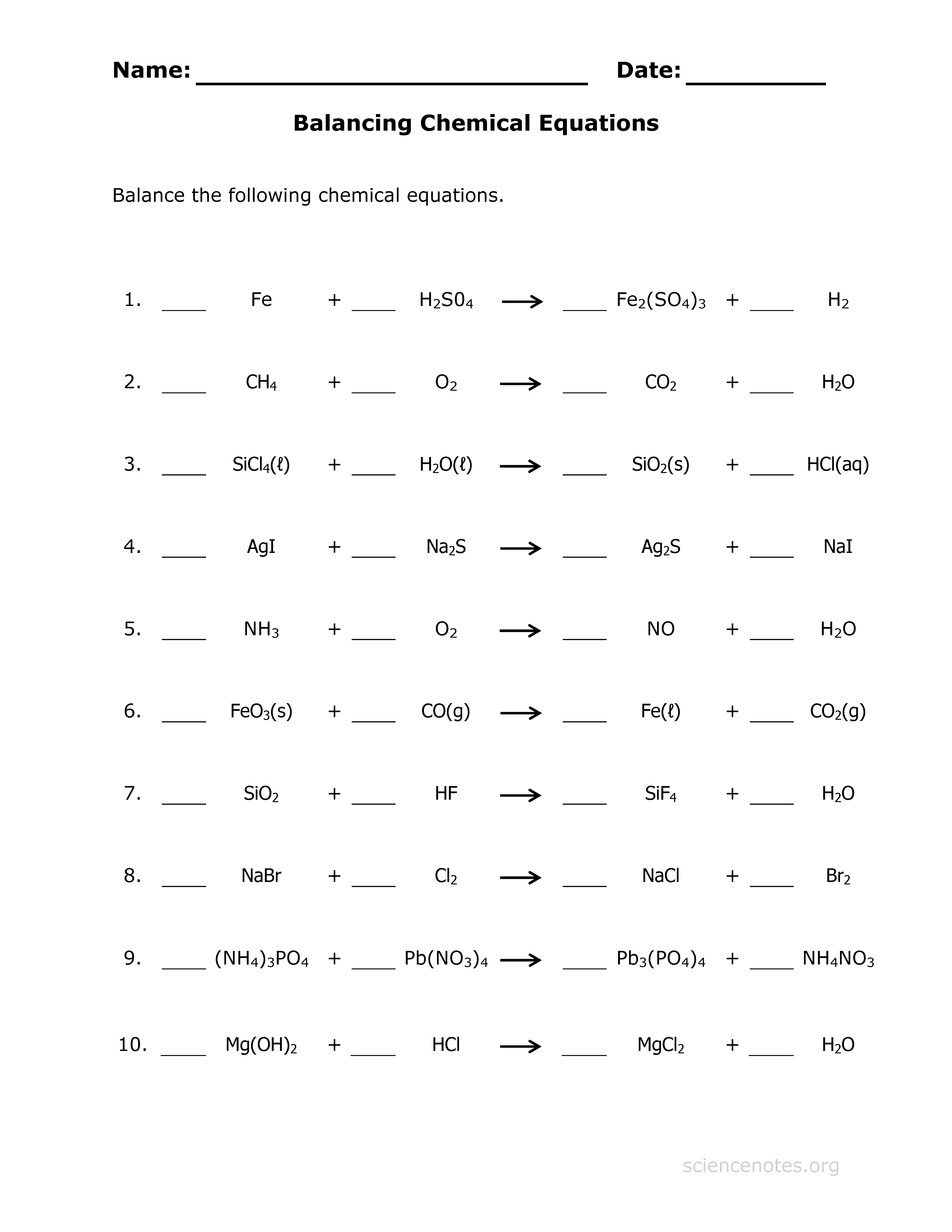
If you're struggling to balance an equation using only whole numbers, don't hesitate to use fractions:
- This can help simplify the balancing process by reducing the coefficients' size.
- After balancing, multiply the entire equation by the least common denominator to get whole numbers.
🧪 Note: Fractional coefficients can make balancing easier initially but remember to convert back to whole numbers for the final equation.
Tip 4: Cross-Check with Stoichiometry

Once you've balanced an equation, use stoichiometry to verify:
- Check the molar mass of each reactant and product.
- Ensure the total mass on both sides is equal, respecting the law of conservation of mass.
Tip 5: Practice, Practice, Practice

Balancing chemical equations, like any skill, requires practice:
- Try different types of reactions to get comfortable with the various balancing techniques.
- Create your own worksheets or use online resources for additional practice.
🔍 Note: There's no substitute for hands-on experience when mastering the art of balancing equations.
In essence, the process of balancing chemical equations is both a science and an art. It combines analytical thinking with practical application, allowing chemists to predict how substances interact, the quantities produced, and the stoichiometry involved. By adhering to these tips, students can refine their balancing techniques, enhancing their overall understanding of chemistry.
Why is it important to balance chemical equations?

+
Balancing ensures that the equation follows the law of conservation of mass, meaning the number of atoms on the reactants’ side equals the number of atoms on the products’ side. This accuracy is crucial for predicting reaction outcomes, calculating yields, and understanding chemical stoichiometry.
Can chemical equations ever be imbalanced?

+
Theoretically, yes, if the law of conservation of mass is not correctly considered or if there are errors in the equation’s formulation. However, properly balanced equations are essential for any meaningful chemical analysis or experimentation.
What if I can’t balance an equation?

+
If you’re having trouble, recheck your work. Ensure you’ve identified all elements, double-check the coefficients, or consider using a different approach like starting with a different element. Sometimes, working through examples or seeking additional help can clarify the process.