Chemistry Atomic Structure Worksheet Answers: Solved!

Understanding Atomic Structure: A Comprehensive Guide

Atomic structure forms the cornerstone of chemistry. It not only defines the properties of atoms but also helps us understand how atoms interact to form compounds. This blog post aims to demystify atomic structure, making it accessible even to those who might not have a deep background in chemistry. Let’s delve into the details.
The Basics of Atomic Theory

Atomic theory has evolved over time, but the core ideas were first solidified by John Dalton, who proposed that all matter is made of tiny indivisible parts called atoms. Today, we understand that atoms are made up of three primary subatomic particles:
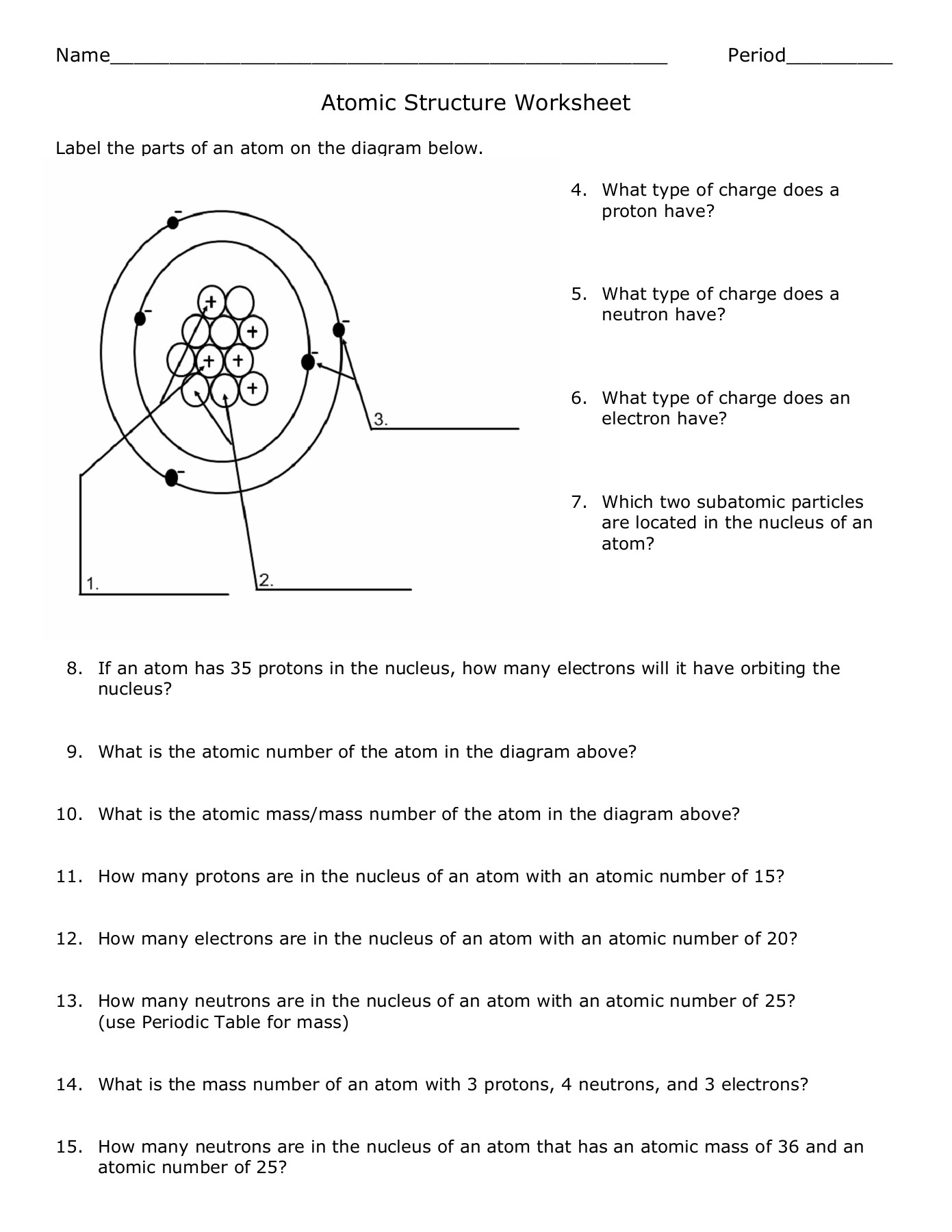
- Protons - Positively charged particles located in the nucleus.
- Neutrons - Neutral particles also found in the nucleus, adding mass but no charge.
- Electrons - Negatively charged particles that orbit the nucleus in electron shells or clouds.
The Nucleus: Heart of the Atom

The nucleus is central to an atom’s identity. Here’s what it consists of:
| Particle | Charge | Mass (amu) |
|---|---|---|
| Proton | +1 | 1.0073 |
| Neutron | 0 | 1.0087 |

The atomic number, which is the number of protons, determines the chemical properties of an element. Isotopes, which have different numbers of neutrons, exhibit the same chemical behavior due to identical proton counts but have different physical properties due to mass differences.
🔬 Note: The number of neutrons in an atom can vary without altering its identity, leading to isotopes.
Electrons: Shells and Orbitals

Electrons occupy energy levels known as shells, which can hold a fixed number of electrons:
- First shell (n=1) can hold up to 2 electrons.
- Second shell (n=2) can hold up to 8 electrons.
- Subsequent shells follow a pattern where the maximum number of electrons in the n-th shell is (2n^2).
Within these shells, electrons reside in orbitals. An orbital can hold up to two electrons and has a specific shape and orientation around the nucleus. Understanding these allows us to predict how atoms bond and form molecules.
Periodic Table and Electron Configuration

The periodic table is organized based on atomic number, with rows (periods) indicating the number of shells and columns (groups) indicating similar chemical properties due to valence electron configurations.
Here is how to determine the electron configuration:
- Find the atomic number to determine the number of electrons.
- Use the Aufbau principle to fill electron shells in order of energy levels, from lowest to highest.
- Remember the exceptions like chromium (Cr) and copper (Cu) where electron configurations deviate from the general pattern.
📝 Note: Valence electrons are key to an element's reactivity. Elements in the same group have the same number of valence electrons.
Practical Applications

Understanding atomic structure has practical implications:
- Chemistry Education: Knowing atomic structure aids in understanding chemical reactions and bonding.
- Technology: Semiconductors in electronics rely on the arrangement of electrons in atoms.
- Medicine: Techniques like nuclear magnetic resonance (NMR) imaging depend on atomic properties.
To wrap up, we've explored the fundamental components of an atom, their interactions, and how they relate to the periodic table. This knowledge not only forms the bedrock of chemistry but also bridges various scientific fields, impacting our understanding of the world and technological advancements.
What is an electron configuration?

+
An electron configuration represents the arrangement of electrons within the atomic orbitals of an atom. It describes how electrons are distributed among various energy levels, subshells, and orbitals in an ordered sequence according to the Aufbau principle, Hund’s rule, and the Pauli Exclusion Principle.
Why are noble gases inert?

+
Noble gases are inert because they have full valence electron shells, which makes them stable and less likely to react with other elements to gain or lose electrons. Their filled outermost energy levels create a state of minimal chemical reactivity.
How does atomic structure affect an element’s properties?
+
The atomic structure, particularly the arrangement of electrons, dictates how an element will behave chemically. The number and arrangement of valence electrons determine an element’s reactivity, its ability to bond with other atoms, and the type of bonds it can form (covalent, ionic, or metallic).
What is the role of neutrons in an atom?

+
Neutrons contribute to the mass of the atom and play a critical role in stabilizing the nucleus. They act like a glue by balancing the repulsive force between positively charged protons, thus preventing nuclear decay in many isotopes.