Master Chemical Reactions: Types of Reactions Worksheet

Understanding chemical reactions is fundamental to mastering chemistry. This guide will delve into various types of chemical reactions, offering you a comprehensive understanding of how elements and compounds interact. Through this exploration, you will encounter key concepts like synthesis, decomposition, single displacement, double displacement, combustion, and more. Each reaction type has its unique characteristics and processes, providing the groundwork for predicting chemical behavior in diverse settings from labs to natural environments.
Types of Chemical Reactions

Synthesis Reactions

A synthesis reaction, often referred to as a combination or composition reaction, is where two or more substances combine to form a single compound. The general form of this reaction is:
A + B → AB
🔍 Note: Synthesis reactions are pivotal in understanding the formation of compounds from simple elements or less complex molecules. A classic example is the formation of water from hydrogen and oxygen gases:
2H2 + O2 → 2H2O
Decomposition Reactions
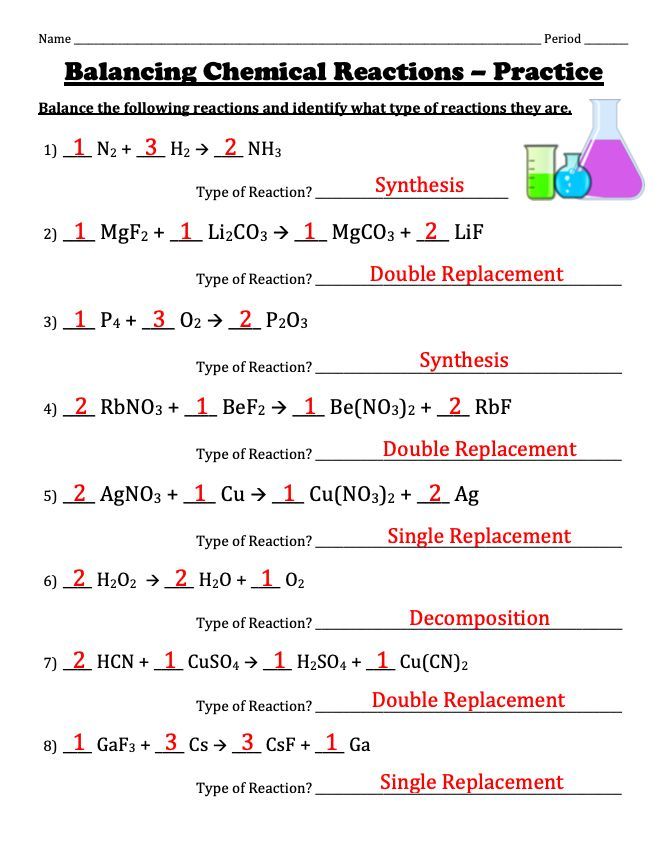
Contrary to synthesis, decomposition reactions involve breaking down a single compound into two or more elements or simpler compounds. The general equation looks like:
AB → A + B
These reactions often require energy input, such as heat, light, or electricity, to occur. Here's an example:
2H2O2 (hydrogen peroxide) → 2H2O + O2
Single Displacement Reactions

In a single displacement reaction, also known as single replacement, one element displaces another element in a compound. This can be represented as:
A + BC → AC + B
These reactions are governed by activity series, where a more reactive element displaces a less reactive one. For instance:
Zn + CuSO4 → ZnSO4 + Cu
Here, zinc displaces copper in copper sulfate.
Double Displacement Reactions

Double displacement reactions occur when the cations and anions of two different compounds switch places, forming two new compounds:
AB + CD → AD + CB
These reactions often result in the formation of a precipitate, gas, or a molecular compound like water:
NaCl + AgNO3 → AgCl(s) + NaNO3
Combustion Reactions

Combustion reactions involve a fuel combining with oxygen to produce oxides and release energy, often in the form of heat and light. The general reaction for hydrocarbon combustion is:
CnHm + O2 → CO2 + H2O
Here's an example with methane:
CH4 + 2O2 → CO2 + 2H2O
🔥 Note: Combustion reactions are exothermic and are crucial in energy production, from burning fuels to cellular respiration.
Acid-Base Reactions

These reactions involve the neutralization of an acid with a base to produce water and a salt:
HCl + NaOH → H2O + NaCl
Redox Reactions

Redox (reduction-oxidation) reactions involve the transfer of electrons between reactants:
- Oxidation involves loss of electrons
- Reduction involves gain of electrons
An example would be the rusting of iron:
4Fe + 3O2 → 2Fe2O3
Here, iron is oxidized to iron(III) oxide, with oxygen acting as the oxidizing agent.
Precipitation Reactions

When two solutions are mixed, and a solid (precipitate) forms, this indicates a precipitation reaction. For instance:
AgNO3 + NaCl → AgCl(s) + NaNO3
AgCl forms as an insoluble solid, precipitating out of the solution.
Recognizing Reaction Types

Understanding the types of reactions helps chemists predict outcomes in chemical processes:
- Look for clues like heat or light production, color changes, formation of gases or precipitates, or changes in the physical state of the reactants or products.
- Recognize reactants and products – understanding the nature of reactants and products can aid in identifying the type of reaction.
- Energy considerations – Endothermic or exothermic reactions can indicate combustion, synthesis, or decomposition reactions.
🧐 Note: While recognizing these signs, remember that some reactions can fit into more than one category, especially redox reactions which often accompany other reactions.
Applications of Chemical Reactions

Chemical reactions are not just laboratory curiosities; they are fundamental to:
- Industry: From pharmaceuticals to agriculture, understanding reactions allows for product development and process optimization.
- Environment: They are key in understanding natural processes like photosynthesis and decomposition, as well as in designing environmentally friendly technologies.
- Medicine: Biochemical reactions underpin life processes, and understanding them aids in drug design and disease treatment.
This exploration of chemical reactions showcases how chemistry underpins so much of our world, from natural processes to the technologies we depend on daily.
What is the difference between synthesis and decomposition reactions?

+
Synthesis reactions combine elements or compounds to form a more complex compound, while decomposition reactions break down a single compound into two or more simpler substances.
Can a reaction be both a redox and an acid-base reaction?
+
Yes, it is possible. For example, in the reaction between hydrochloric acid and sodium hydroxide, there is an acid-base neutralization, but also a redox reaction as sodium loses an electron to become Na+ and H+ in HCl gains an electron.
How do you predict the products of a reaction?
+Predicting products involves understanding the type of reaction, applying the rules of ion exchange in double displacement reactions, considering the activity series in single displacement, and recognizing common patterns of synthesis, decomposition, or combustion reactions.