Master 5 Common Chemical Reaction Types Easily

Have you ever tried to grasp the complexities of chemistry, only to find yourself tangled in the seemingly endless array of reactions and equations? Fear not, for understanding chemical reactions doesn't have to be a Herculean task. With a bit of knowledge and practice, you can easily master the five common types of chemical reactions. Today, we'll delve into each of these types, providing you with the tools to identify and predict them with confidence.
Combination Reaction: When Elements Unite


A combination reaction, also known as a synthesis reaction, is where two or more substances (usually elements or simple compounds) combine to form a single, more complex compound. This is one of the simplest types of chemical reactions to understand:
- Hydrogen + Oxygen -> Water
- 2H2(g) + O2(g) -> 2H2O(l)
- Iron + Sulfur -> Iron(II) Sulfide
- Fe(s) + S(s) -> FeS(s)
These reactions often release energy, as forming bonds usually lowers the overall energy of the system.
Decomposition Reaction: Breaking Down Complex Substances


Decomposition reactions, on the other hand, involve a single compound breaking down into two or more simpler substances. These reactions require energy to break the chemical bonds, often in the form of heat, electricity, or light:
- Water -> Hydrogen + Oxygen
- 2H2O(l) -> 2H2(g) + O2(g)
- Calcium Carbonate -> Calcium Oxide + Carbon Dioxide
- CaCO3(s) -> CaO(s) + CO2(g)
Note that decomposition reactions can be reversed by combination reactions under appropriate conditions.
Single Displacement (Replacement) Reaction: Swapping Partners


In a single displacement reaction, one element replaces another in a compound. Here's how they generally look:
- Metal displacement: Zn(s) + CuSO4(aq) -> ZnSO4(aq) + Cu(s)
- Hydrogen displacement: 2Na(s) + 2H2O(l) -> 2NaOH(aq) + H2(g)
- Halogens: Cl2(g) + 2KBr(aq) -> 2KCl(aq) + Br2(aq)
The key to predicting these reactions is understanding the activity series of metals, which helps determine which elements will displace others.
Double Displacement (Replacement) Reaction: Exchanging Ions
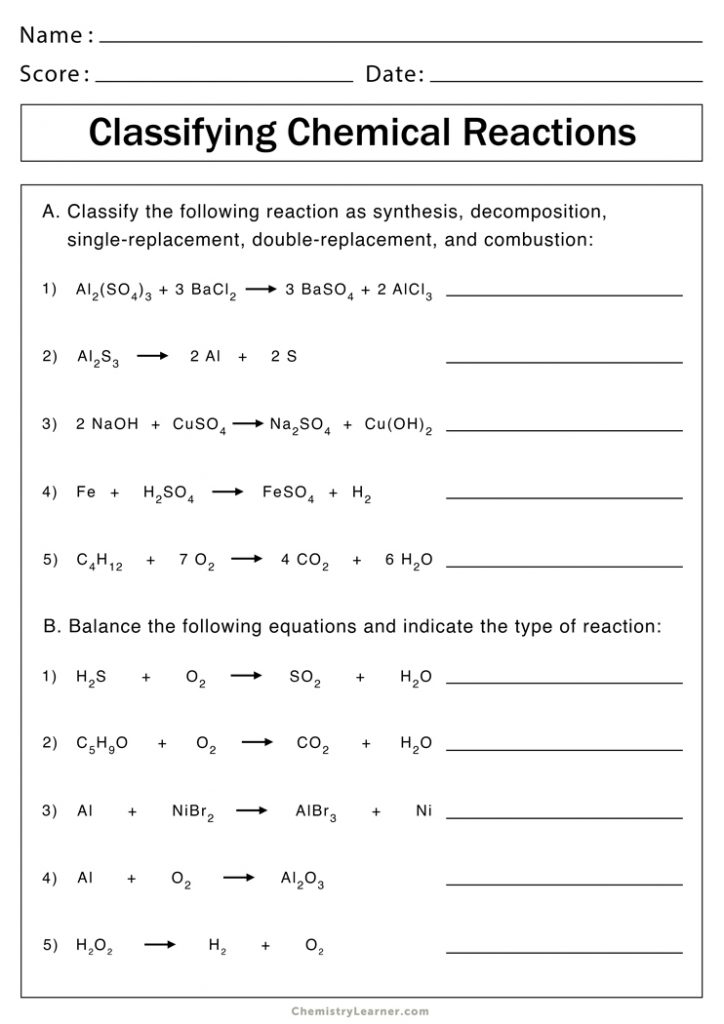

In double displacement reactions, ions from two different compounds switch places. The outcome typically results in either the formation of an insoluble precipitate, a gas, or a molecular compound like water:
| Reactants | Products | Equation |
|---|---|---|
| Silver nitrate + Sodium chloride | Silver chloride + Sodium nitrate | AgNO3(aq) + NaCl(aq) -> AgCl(s) + NaNO3(aq) |
| Lead(II) nitrate + Potassium iodide | Lead(II) iodide + Potassium nitrate | Pb(NO3)2(aq) + 2KI(aq) -> PbI2(s) + 2KNO3(aq) |

Knowing the solubility rules is crucial here, as it helps predict which reactions will yield a solid or a gas.
Combustion Reaction: Burning Brightly


Combustion reactions involve a substance, usually containing carbon and hydrogen, reacting with oxygen, often producing carbon dioxide, water, and heat:
- Methane: CH4(g) + 2O2(g) -> CO2(g) + 2H2O(g)
- Glucose: C6H12O6(s) + 6O2(g) -> 6CO2(g) + 6H2O(l)
Combustion reactions are exothermic, meaning they release heat, and are the basis for most energy production in our modern world.
🔬 Note: Not all reactions fit neatly into one category. Some can be combinations of the above types, and external factors like catalysts or temperature changes can influence reaction pathways.
To master these chemical reactions, it's crucial to practice, observe, and understand the conditions under which they occur. Remember, chemistry is as much about understanding patterns and exceptions as it is about memorizing facts. Here are some key tips to remember:
- Identify the reactants and predict the products based on the type of reaction.
- Balance the chemical equations to conserve mass.
- Use the activity series, solubility rules, and energy changes to predict reaction outcomes.
With a firm grasp on these five common types of chemical reactions, you'll find chemistry not only becoming more manageable but also more fascinating. Each reaction type offers a unique glimpse into how atoms and molecules interact, rearrange, and transform to create new substances, fuel our cars, power our cities, and sustain life itself.
What are the conditions for a combination reaction to occur?

+
Combination reactions typically occur when the elements or compounds have a strong affinity for each other, often requiring a release of energy (exothermic reaction) or specific conditions like heat or pressure to initiate the reaction.
Can decomposition reactions be reversed?

+
Yes, decomposition reactions can often be reversed through combination reactions under suitable conditions, where the products are combined again to form the initial compound.
How do I predict which element will displace another in a single displacement reaction?

+
Use the activity series of metals to determine displacement. A metal higher on the activity series will displace one lower, taking its place in the compound. For non-metals, a similar series exists for halogens.