5 Tips to Ace Chemical Equations Worksheet Answers

🎓 Note: Remember, the key to mastering chemical equations is a combination of understanding the principles and a healthy dose of practice. Here are 5 tips to help you excel in this area:
Practice, Practice, Practice

There’s no shortcut to learning how to balance chemical equations effectively. Just like any other skill, the more you practice, the more proficient you get. Here are some ways to enhance your practice:
- Use Workbooks or Textbooks: These provide a structured approach to balancing equations.
- Online Exercises: Numerous websites offer interactive tools where you can balance chemical equations and instantly get feedback.
- Flashcards: Create flashcards of common reactions to get quicker at recognizing common patterns.

Understand the Law of Conservation of Mass

Balancing chemical equations involves obeying the law of conservation of mass, where the total mass of reactants equals the total mass of products. Here’s how to apply it:
- Count the number of atoms for each element on both sides of the equation.
- Use coefficients to balance the number of atoms on both sides, ensuring no atoms are created or destroyed.
🧪 Note: Remember, subscripts cannot be changed as they define the chemical identity of compounds. Only coefficients can be altered.
Recognize Common Reaction Types
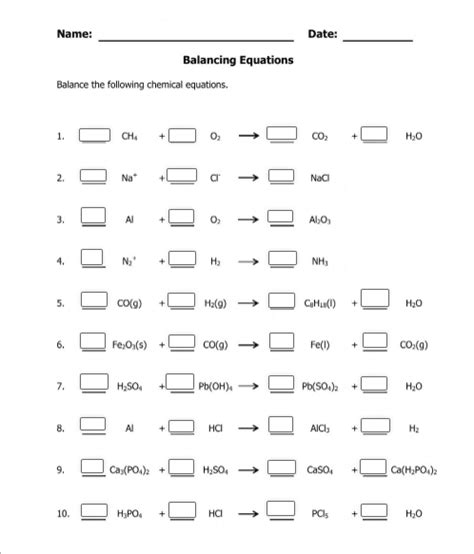
Chemical equations often follow predictable patterns. Here are some common types:
- Combustion Reactions: Hydrocarbon + O2 → CO2 + H2O
- Decomposition Reactions: AB → A + B
- Synthesis Reactions: A + B → AB
- Single Replacement Reactions: A + BC → AC + B
- Double Replacement Reactions: AB + CD → AD + CB
| Reaction Type | Example |
|---|---|
| Combustion | CH4 + 2O2 → CO2 + 2H2O |
| Decomposition | 2H2O → 2H2 + O2 |
| Synthesis | 2Mg + O2 → 2MgO |

Check Your Work

After balancing an equation, it’s crucial to:
- Double-check the count of each element to ensure they are the same on both sides.
- Examine if the charges balance if you’re working with ionic equations.
- Review the entire equation for any other oversights.
🔍 Note: Mistakes often happen when we assume our initial guesses are correct. A second look can save valuable points!
Seek Feedback

Even with the best practices, sometimes you need guidance. Here’s how to get feedback:
- Ask your teacher or tutor for help if you’re stuck on a problem.
- Join study groups where you can exchange methods and correct each other’s work.
- Use forums or online communities for real-time problem-solving assistance.
Putting effort into mastering chemical equations isn’t just about getting the correct answer; it’s also about understanding the intricate dance of elements and compounds, ensuring we follow nature’s laws while delving into chemistry’s depths.
Why is balancing chemical equations important?

+
Balancing chemical equations is critical because it adheres to the law of conservation of mass, ensuring that the number of atoms of each element remains constant throughout the reaction, representing real-world chemical reactions accurately.
How do I know if I’ve balanced the equation correctly?

+
To check if your equation is balanced, count the atoms of each element on both sides of the reaction. If the numbers are equal, the equation is balanced. Also, ensure that the charges balance if you’re dealing with ionic species.
What if I can’t balance the equation?

+
If you’re struggling to balance an equation, consider:
- Rechecking the equation for any copy errors.
- Seeking help from textbooks or online resources.
- Remembering common reaction patterns that might help balance the equation.