5 Essential Tips for Chemical Bonding Worksheet Answers

Mastering the intricacies of chemical bonding is fundamental for any student of chemistry. Bonds form the backbone of how atoms interact to create molecules, determine the properties of compounds, and influence reactivity. If you're working through chemical bonding worksheet answers, here are five essential tips that will not only help you better understand the material but also make you more adept at tackling problems.
1. Understand the Basic Concepts

Before diving into specific worksheet answers, ensure you have a solid foundation in the basics:
- Atomic Structure: Know the roles of protons, neutrons, and electrons, especially how electron configurations influence bonding.
- Types of Bonds: Be clear on ionic, covalent, and metallic bonds. Understand how they form and their characteristics.
- Valence Shell Electron Pair Repulsion (VSEPR): This model will help predict molecular geometry which is often a part of bonding worksheets.
Key Concepts:

- Electronegativity: How it determines bond polarity.
- Lewis Structures: Drawing them to show bonding and non-bonding electrons.
- Octet Rule: An atom’s tendency to achieve a noble gas configuration.
2. Practice with Different Elements

When answering questions in a chemical bonding worksheet:
- Try bonding various elements together, focusing on different electron configurations.
- Work through examples involving both main group and transition metals.
- Understand the differences when dealing with elements from different groups and periods in the periodic table.
🔗 Note: Elements from different groups have different tendencies for bonding. Understanding the periodic trends will help predict bond types.
3. Use Diagrams and Visual Aids

Visual representation is key to mastering chemical bonding:
- Draw Lewis dot structures or electron dot diagrams to visualize bonding.
- Use 3D models or stick-and-ball kits to understand molecular shapes.
- Graph paper or computer simulations can help plot the spatial arrangement of atoms.
4. Approach with a Systematic Method
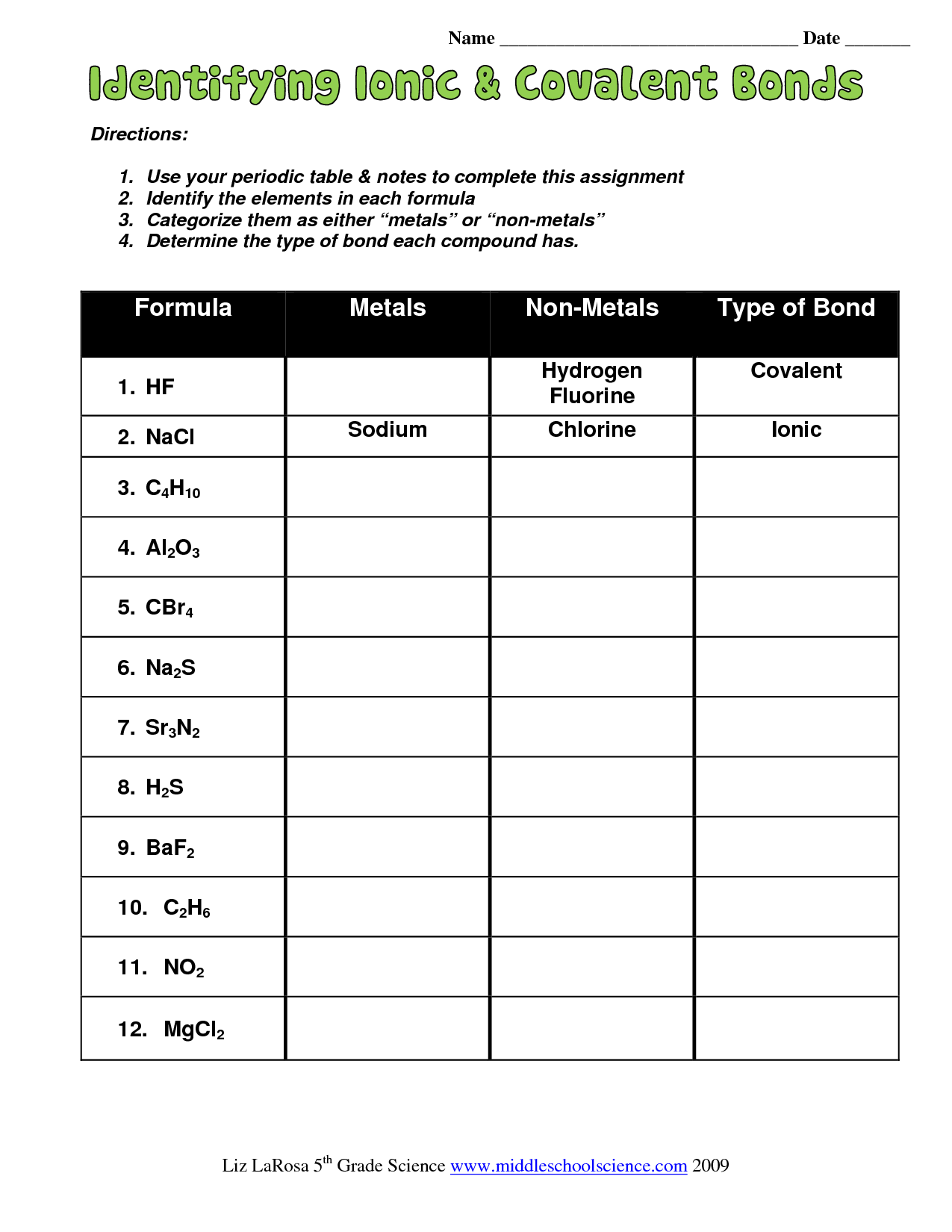
When solving problems on a worksheet:
- Identify the elements involved and their electron configurations.
- Determine the likely bond type based on electronegativity differences.
- Draw the Lewis structure, considering the octet rule.
- Identify any formal charges or resonance structures if applicable.
- Use VSEPR theory to predict molecular shape and polarity.
5. Connect Concepts

Don't approach chemical bonding in isolation:
- Relate bonding to compound properties like melting point, solubility, and conductivity.
- Understand how bonding influences reaction mechanisms and molecular reactivity.
- Explore the relationship between bonding and energy considerations like bond enthalpy and stability.
In summary, mastering chemical bonding worksheet answers requires a strong grasp of basic concepts, consistent practice with different elements, visual aids for better understanding, a systematic approach to problem-solving, and the ability to connect these concepts to the broader context of chemistry. With these tips, you'll be well on your way to excelling in chemistry.
What is the easiest way to predict if a bond is ionic or covalent?

+
The easiest way to predict the type of bond between two elements is by considering the electronegativity difference. If the electronegativity difference is greater than 1.7, the bond is likely to be ionic; if it’s between 0.5 and 1.7, it’s considered polar covalent; and less than 0.5, it’s non-polar covalent.
Why is the octet rule important in bonding?

+
The octet rule is important because it states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons, which mimics the electron configuration of noble gases. This stability drives chemical reactions and bonding.
Can I use the same approach for all elements when doing a bonding worksheet?

+
While many elements follow general rules, there are exceptions, especially with transition metals or elements with expanded octets. Always consider the specific electron configurations and periodic trends when bonding.