5 Essential Charles Laws Answers for Students
Understanding Charles's Law is pivotal in the study of Physics, particularly when you delve into the world of thermodynamics and gas behavior. Here, we offer five essential answers to common questions about Charles's Law, ensuring a solid foundation for students in science.
What Is Charles’s Law?

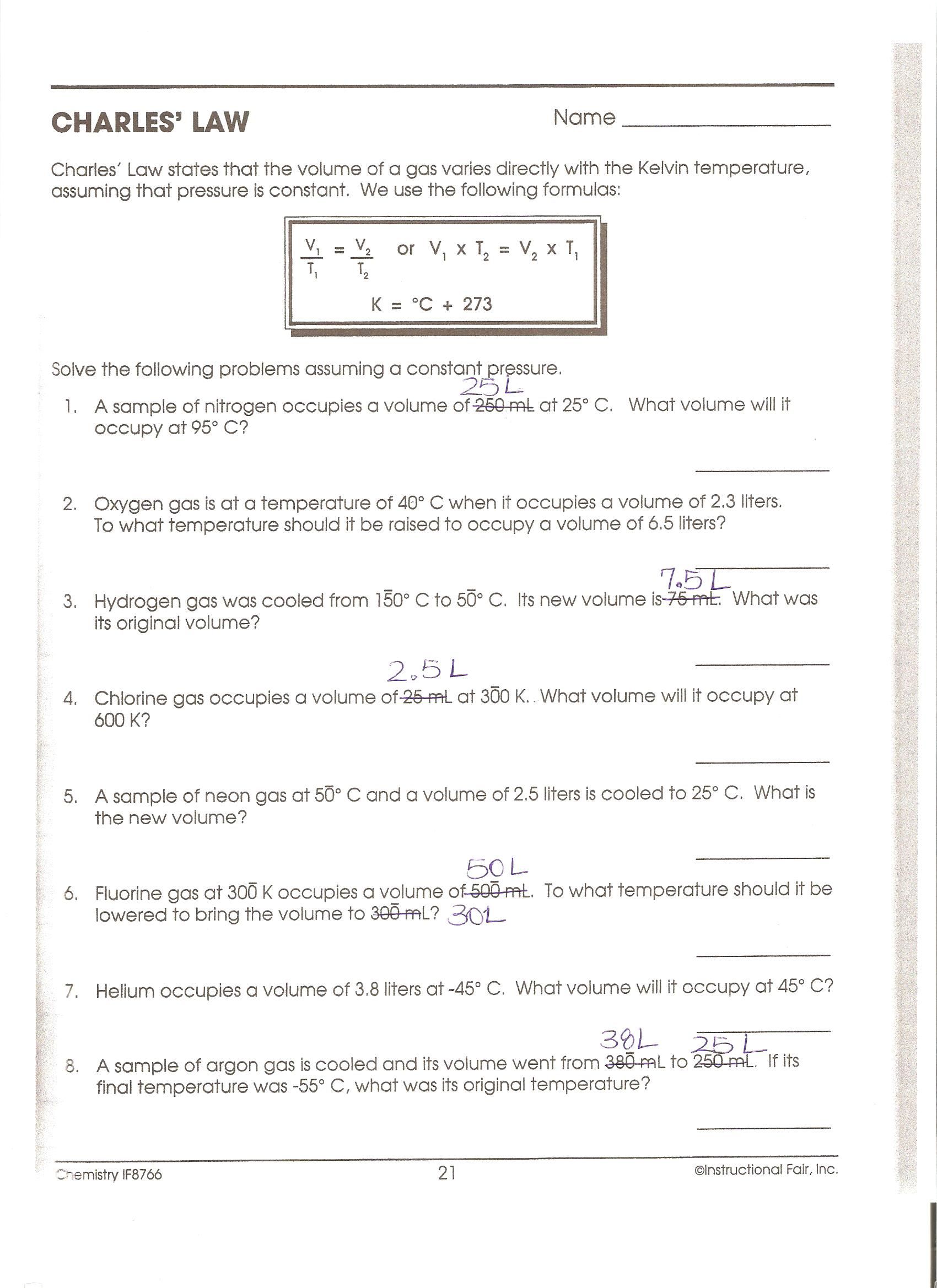
Charles’s Law, named after the French scientist Jacques Charles, elucidates the relationship between the volume and the temperature of a gas, keeping the pressure constant. Mathematically, it is expressed as:
- V1/T1 = V2/T2
This formula indicates that if the volume of a gas doubles, the temperature must double as well, assuming the pressure remains the same. It’s crucial for understanding gas behavior under different conditions.

Why Is Charles’s Law Important?

Charles’s Law is not just theoretical; it has numerous practical applications:
- Hot Air Balloons: The expansion of air when heated allows balloons to rise.
- Automobile Engines: It explains how increasing the temperature in an engine cylinder can increase the volume, which is crucial for engine efficiency.
- Refrigeration: Understanding how cooling reduces the volume of gases is key to refrigeration technology.
How Does Charles’s Law Differ from Boyle’s Law?

While both Charles’s and Boyle’s Laws are fundamental gas laws, they describe different phenomena:
- Boyle’s Law: States that the volume of a gas is inversely proportional to the pressure at constant temperature (P1V1 = P2V2).
- Charles’s Law: Indicates that the volume of a gas is directly proportional to the absolute temperature at constant pressure (V1/T1 = V2/T2).
💡 Note: The difference between these laws lies in what remains constant during the changes in volume and temperature.
Can Charles’s Law Be Applied to All Gases?

Charles’s Law is primarily derived for an ideal gas, but it can be applied to real gases under:
- Low pressure
- High temperatures
However, under extreme conditions (very high pressures or very low temperatures), real gases deviate from ideal behavior due to intermolecular forces and molecular volumes.
| Condition | Ideal Gas | Real Gas |
|---|---|---|
| Low Pressure | Follows Charles’s Law | Follows approximately |
| High Temperature | Follows Charles’s Law | Follows approximately |
| High Pressure or Low Temperature | Follows Charles’s Law | Deviates significantly |

What Are the Limitations of Charles’s Law?

While Charles’s Law is a powerful tool in understanding gas behavior, it does have limitations:
- Ideal Gas Assumption: It assumes the gas is ideal, which isn’t true for all conditions.
- Constant Pressure: The law assumes that pressure does not change, which is often not the case in real-world scenarios.
- Does Not Account for:
- Intermolecular forces
- Molecular volume
In summary, Charles's Law provides a fundamental understanding of how gas volume changes with temperature at constant pressure. It has practical applications in everyday technology and has shaped our understanding of thermodynamics. Yet, while invaluable, its use is most accurate under specific conditions where real gases behave more like ideal gases.
What is the relationship between volume and temperature according to Charles’s Law?

+
According to Charles’s Law, the volume of a gas is directly proportional to its absolute temperature at constant pressure. This means as the temperature increases, the volume of the gas will also increase, and vice versa.
Can Charles’s Law be used in weather forecasting?

+
Yes, Charles’s Law can influence weather forecasting by helping predict the expansion or contraction of gases in the atmosphere. For instance, understanding temperature changes can help predict how air masses behave, contributing to weather pattern predictions.
What happens if you violate Charles’s Law by not keeping pressure constant?

+
If the pressure isn’t constant, the relationship between volume and temperature changes. The gas will still expand or contract with temperature changes, but not according to the exact proportions specified by Charles’s Law.
How does Charles’s Law relate to the ideal gas law?

+
Charles’s Law is a component of the ideal gas law, which combines Charles’s Law with Boyle’s Law and Gay-Lussac’s Law to give PV = nRT, where P is pressure, V is volume, n is the amount of substance, R is the ideal gas constant, and T is the absolute temperature.