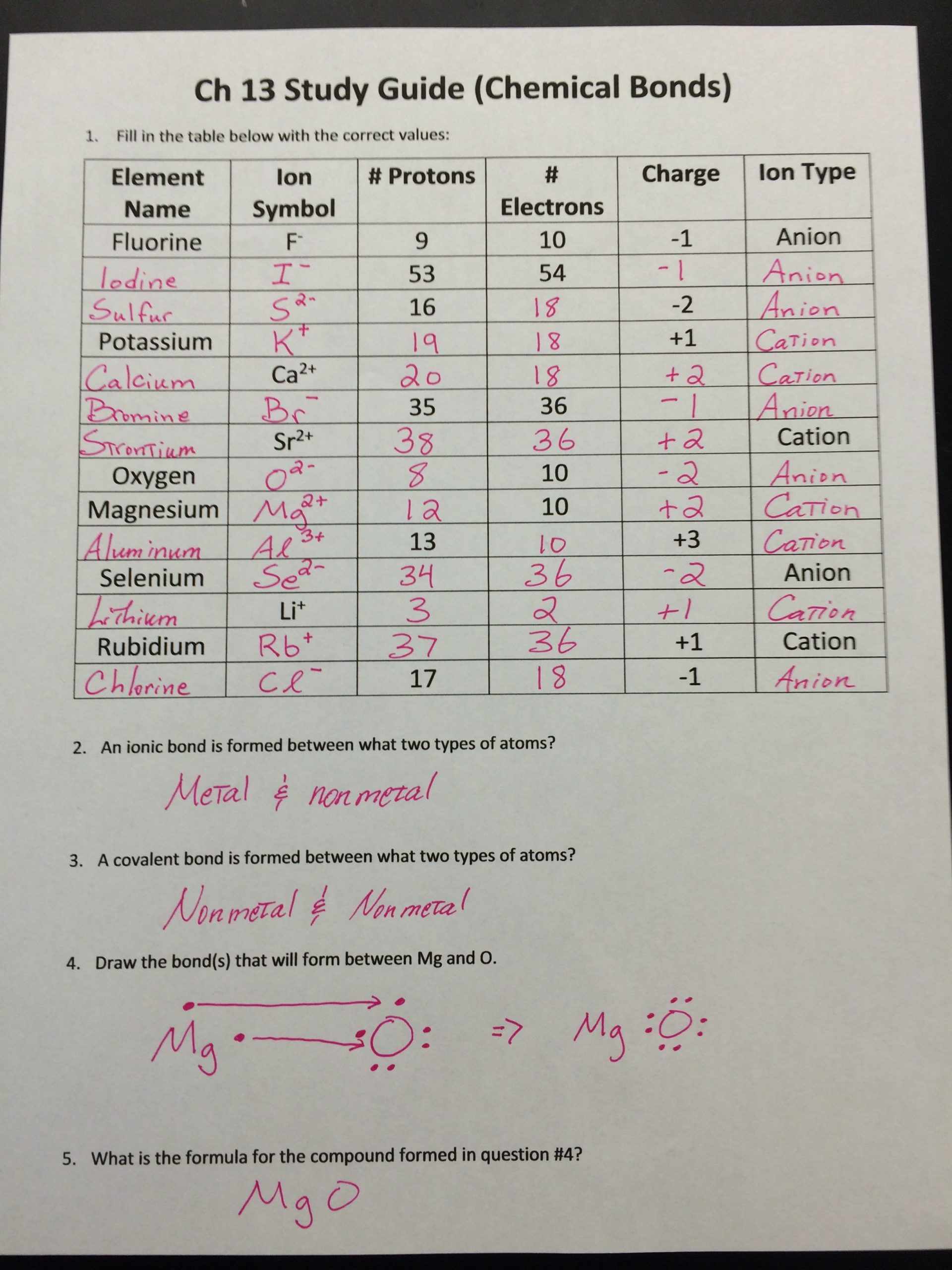
Charges Of Ions Worksheet Answer Key
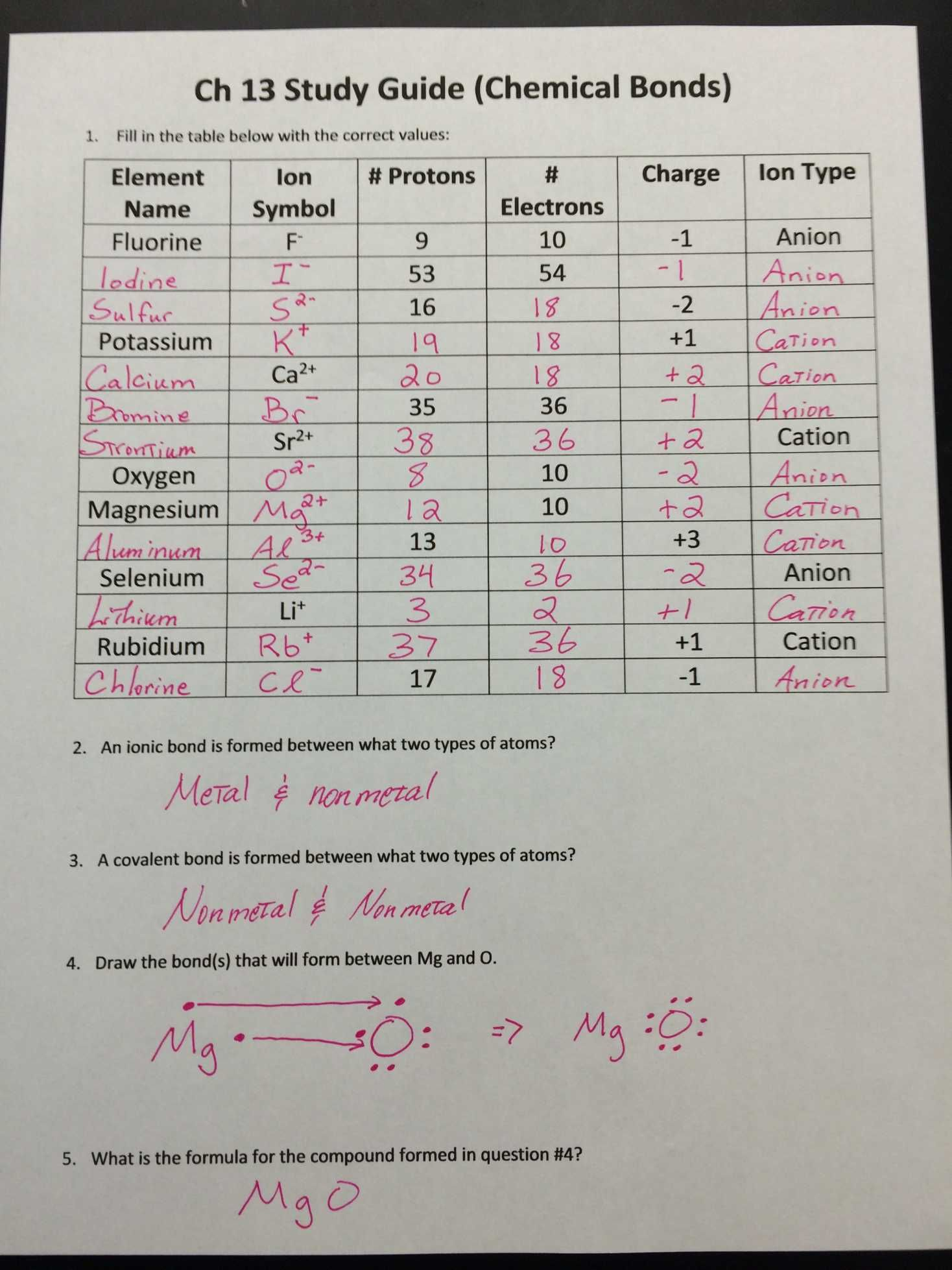
The concept of ions and their charges can be quite fundamental in understanding chemistry, particularly in areas involving chemical reactions, bonding, and electrochemistry. This blog post will delve into the intricacies of understanding the charges of ions, provide detailed explanations, and include practical worksheets along with their answer keys. Here, we'll explore how to identify the charges on common ions and address some typical scenarios you might encounter in classroom or lab settings.
Understanding Ion Formation
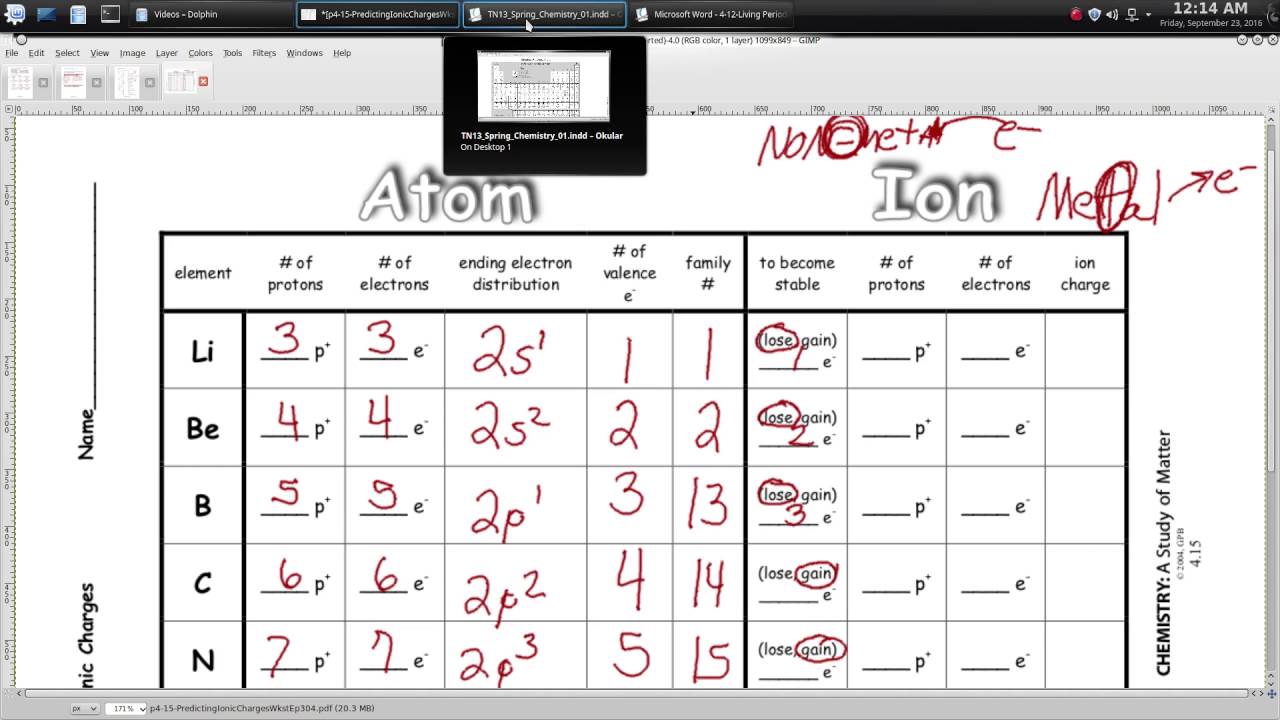
Before diving into specific charges, it's essential to grasp how ions are formed:
- Elements gain or lose electrons to achieve a full outer shell which is either a noble gas configuration or a stable electron octet.
- Cations are positively charged ions formed when an atom loses one or more electrons.
- Anions are negatively charged ions formed when an atom gains one or more electrons.

⚗️ Note: The charge of an ion is determined by the number of electrons it gains or loses. For example, losing one electron results in a +1 charge, while gaining one electron gives a -1 charge.
Common Ion Charges
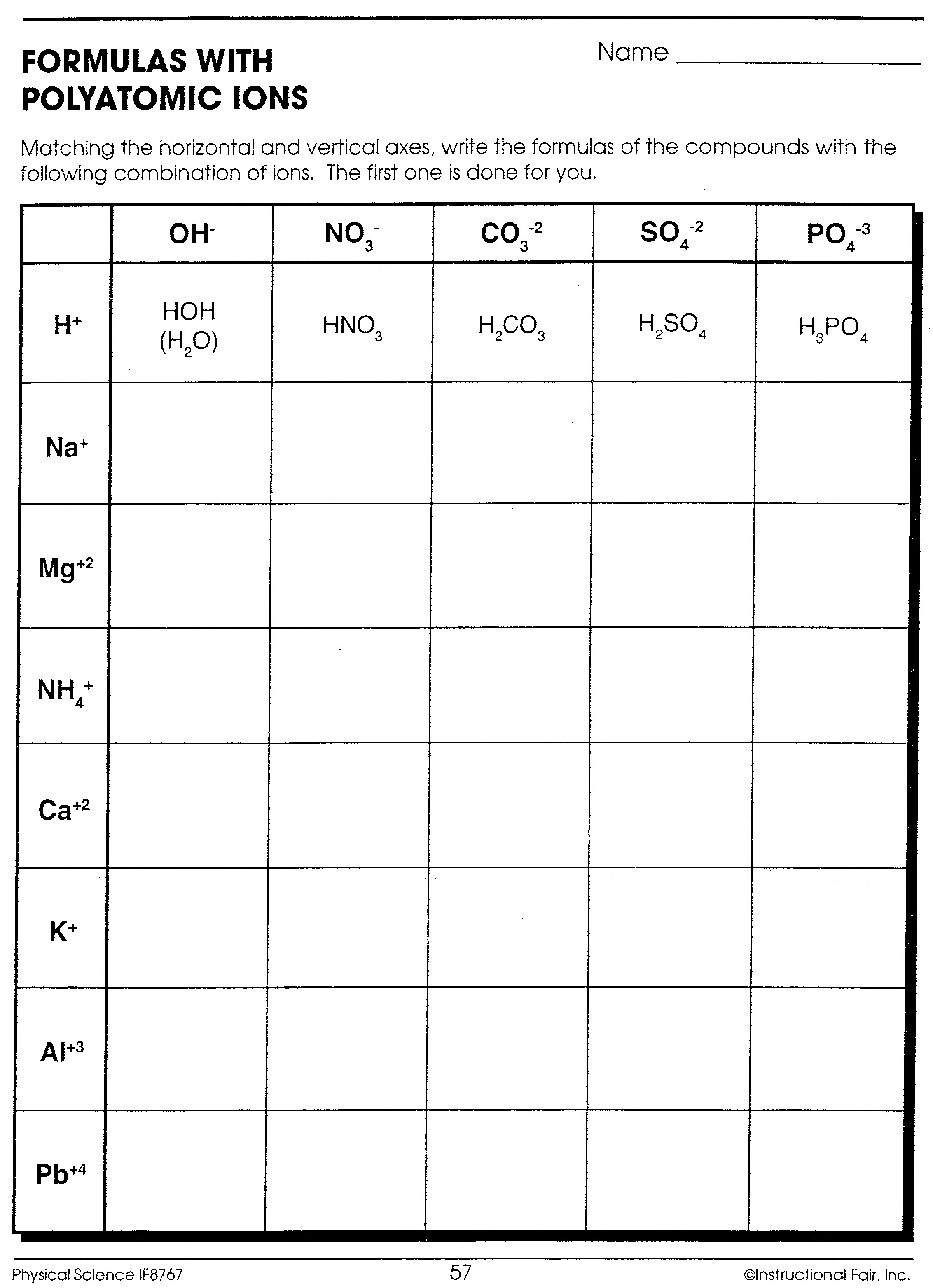
| Element | Charge | Example |
|---|---|---|
| Hydrogen (H) | +1 | H+ |
| Helium (He) | 0 | N/A (Noble Gas) |
| Lithium (Li) | +1 | Li+ |
| Beryllium (Be) | +2 | Be2+ |
| Boron (B) | +3 | B3+ |
| Carbon (C) | +4 or -4 | C4+, C4- |
| Nitrogen (N) | -3 | N3- |
| Oxygen (O) | -2 | O2- |
| Fluorine (F) | -1 | F- |
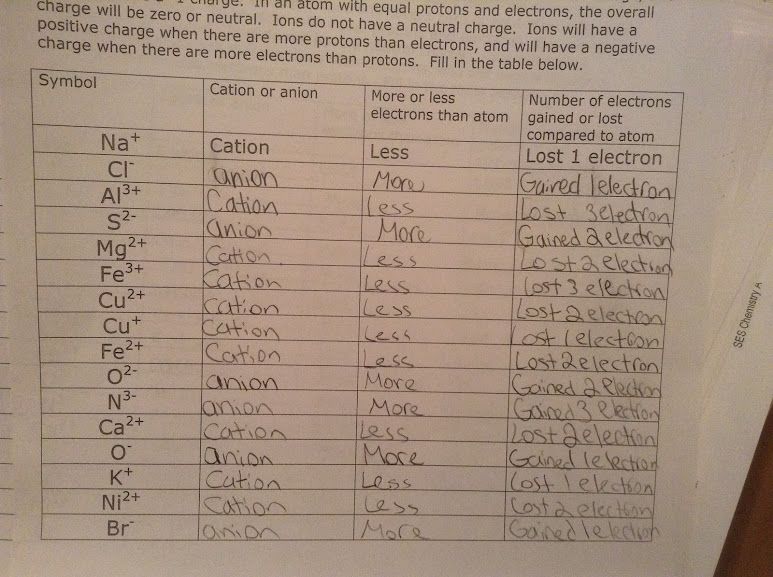
Here's a detailed look at how these charges are commonly encountered:
- Group 1 (Alkali Metals): Always form +1 cations.
- Group 2 (Alkaline Earth Metals): Always form +2 cations.
- Group 13: Usually form +3 cations.
- Group 15 (Nitrogen Group): Often form -3 anions.
- Group 16 (Oxygen Group): Tend to form -2 anions.
- Group 17 (Halogens): Form -1 anions.
💡 Note: Transition metals can have variable charges, but for general purposes, we use the most common charges unless specified.
Worksheet and Answer Key
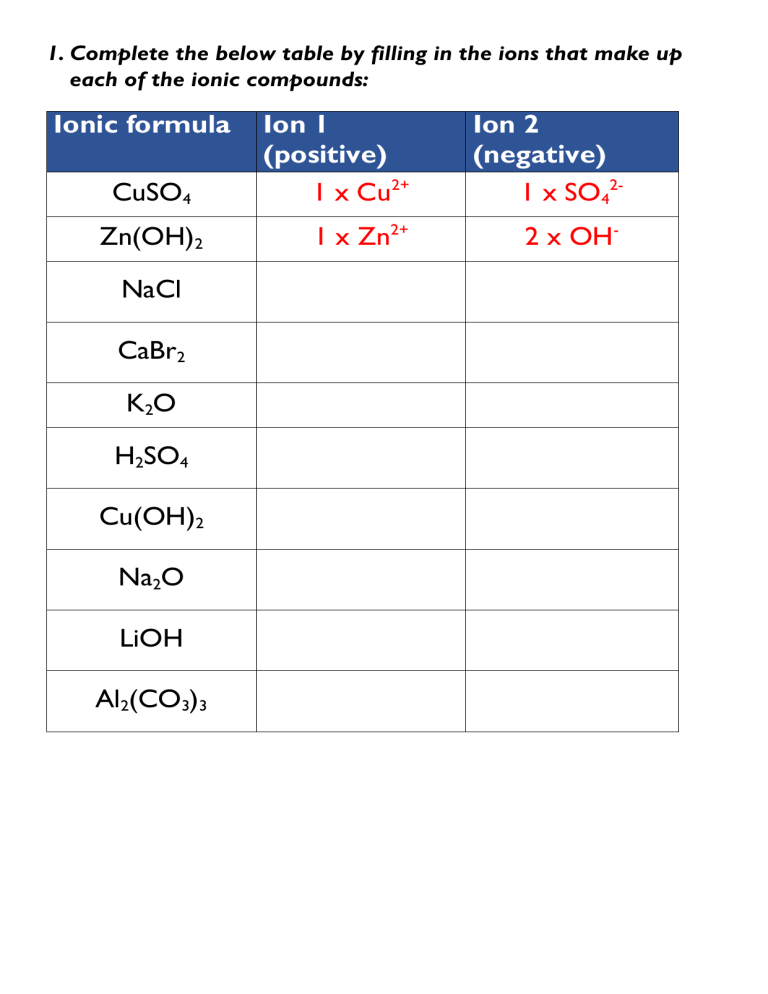
To help solidify your understanding of ion charges, here's a worksheet that you might encounter in a chemistry class:
Worksheet: Identify the charge of the following ions: 1. Sodium (Na) 2. Calcium (Ca) 3. Phosphorus (P) 4. Sulfur (S) 5. Iron (Fe)
Here is the answer key for the worksheet:
Answer Key: 1. Sodium (Na): +1 2. Calcium (Ca): +2 3. Phosphorus (P): -3 4. Sulfur (S): -2 5. Iron (Fe): Commonly +2 or +3 (Fe2+ or Fe3+)
🔍 Note: The charges given for Fe are the most common ones. Transition metals like Iron can have multiple oxidation states.
Advanced Ionic Charge Scenarios
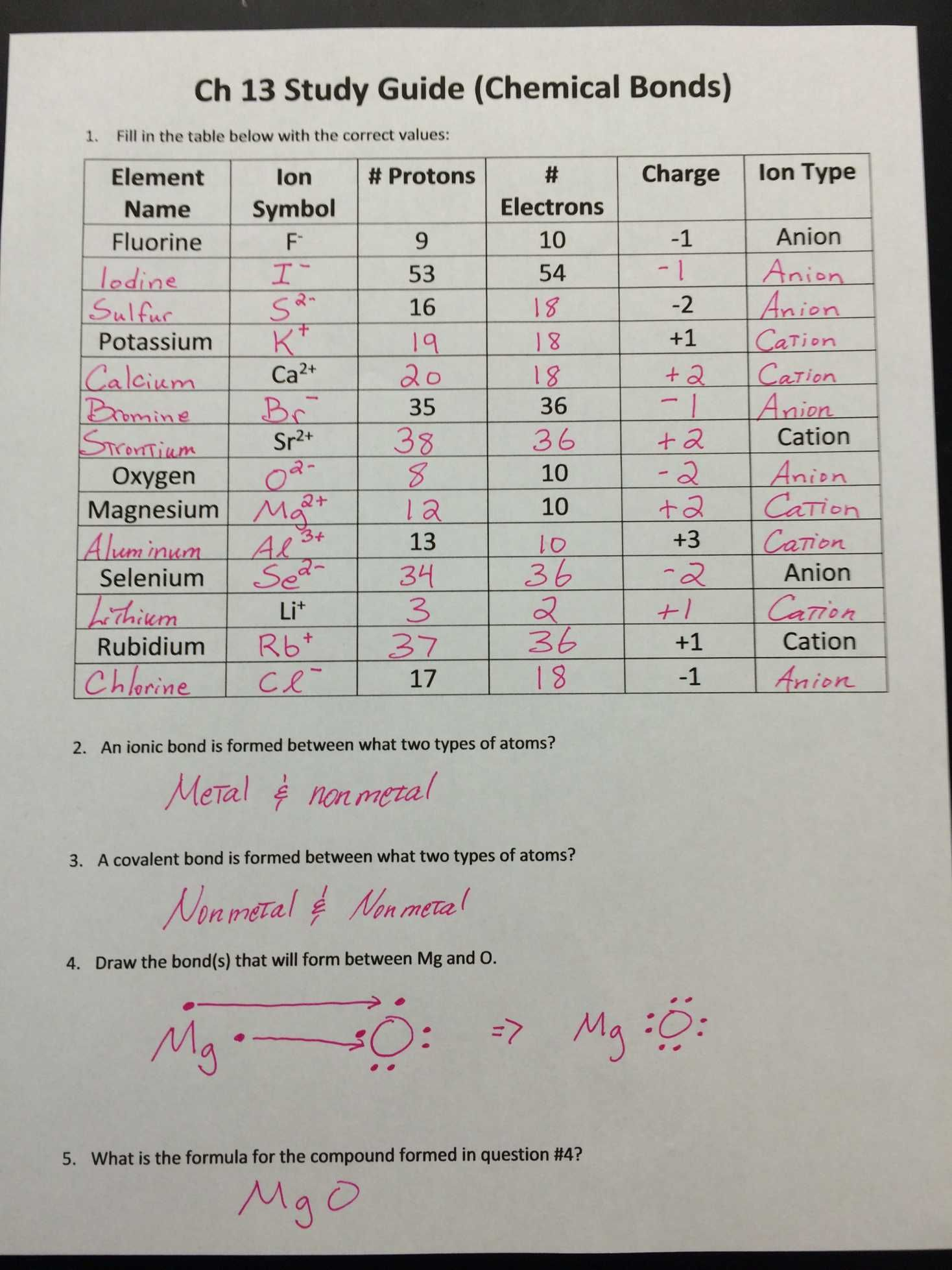
Sometimes, the scenario gets a bit more complex:
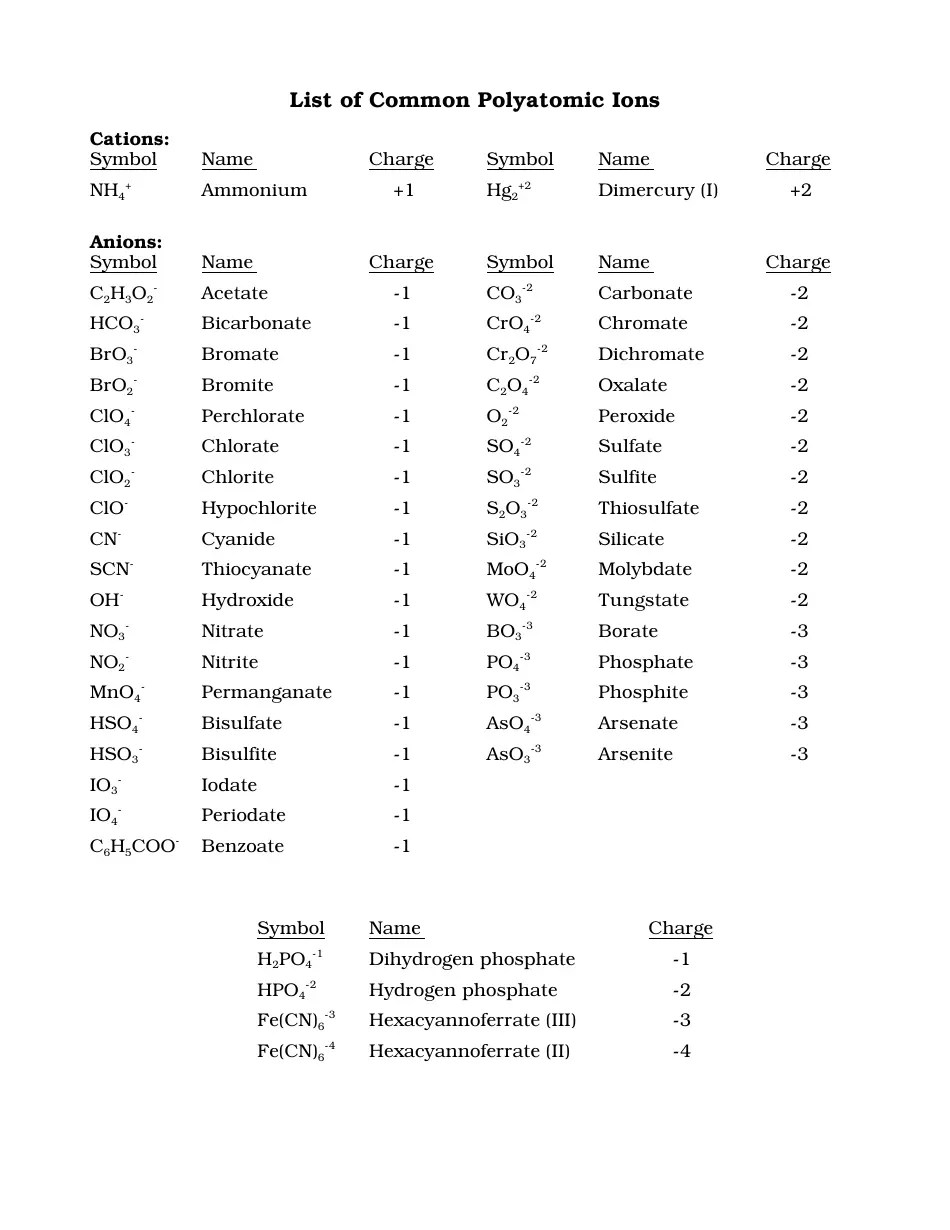
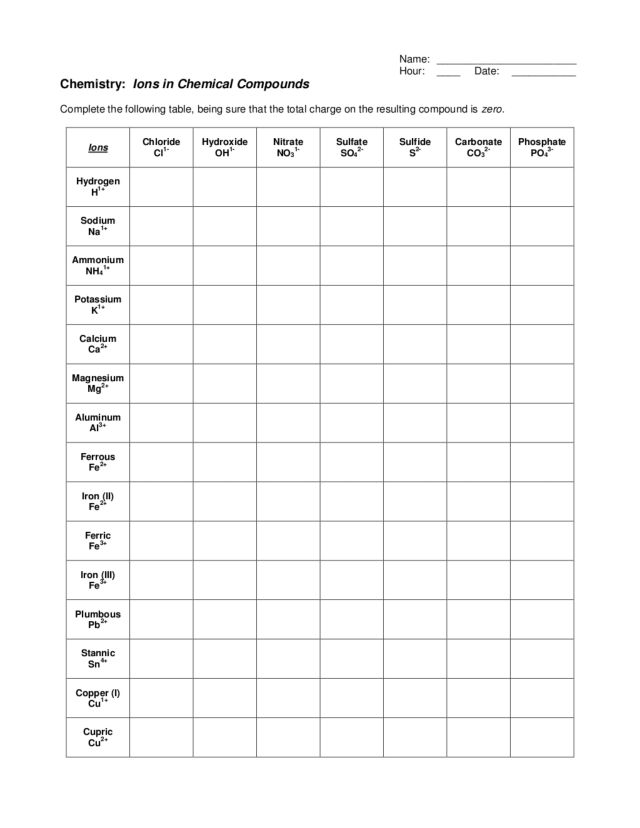
- Polyatomic Ions: These are groups of atoms that carry a charge. Examples include NH4+ (Ammonium) and SO42- (Sulfate).
- Variable Charges: Transition metals and some main group elements can exhibit variable charges. Determining the charge in these scenarios often requires context from the compound or the reaction environment.

To wrap up our exploration of ion charges, understanding these foundational principles is crucial for progressing in chemistry. This knowledge is not just academic; it applies directly to real-world applications, from pharmaceuticals to environmental science. By mastering these basic concepts, you're well on your way to deeper chemical understanding and more complex applications in various fields.
Why do elements form ions?
+
Elements form ions to achieve a full outer shell of electrons, either through gaining or losing electrons, aiming for stability through a noble gas configuration.
How can one predict the charge of an ion?
+
The charge can often be predicted by looking at the element’s position in the periodic table. Groups 1-2 tend to lose electrons to form positive ions, and Groups 15-17 tend to gain electrons to form negative ions.
What are polyatomic ions?
+
Polyatomic ions are ions that consist of two or more atoms covalently bonded together that carry a net charge. Examples include NH4+ and CO32-.
Can an element have more than one ionic charge?
+
Yes, especially transition metals can exhibit variable charges. For example, Iron can be either Fe2+ or Fe3+.
What role do ions play in biological systems?
+
Ions are crucial in biological systems; they are involved in enzyme functions, maintaining electrolyte balance, nerve impulses, and the structure of cellular components like DNA and proteins.