Mastering Ion Charges: Essential Worksheet Guide

Delving into the world of chemistry, one often encounters the fundamental challenge of understanding ion charges. These charges are not just numerical values but keys to unlocking the mysteries of chemical bonding and reactivity. In this comprehensive guide, we aim to explore ion charges, equip you with practical knowledge, and provide tools like worksheets to enhance your understanding. Let's embark on this educational journey to master ion charges!
Why Ion Charges Matter

Ion charges are pivotal for several reasons:
- Chemical Bonding: They dictate how atoms bond to form compounds.
- Chemical Reactivity: The charge influences how an ion will react with other substances.
- Electrolytes in Solutions: Understanding ion charges helps in interpreting the behavior of electrolytes.
Understanding Ions and Charges
An ion is an atom or molecule that has gained or lost one or more of its valence electrons, giving it a net electric charge. Here’s how you identify ions:
- Anion: A negatively charged ion formed by the gain of electrons. For example, when oxygen gains two electrons to form the oxide ion (O²⁻).
- Cation: A positively charged ion created by the loss of electrons. For instance, sodium loses an electron to become a sodium cation (Na⁺).

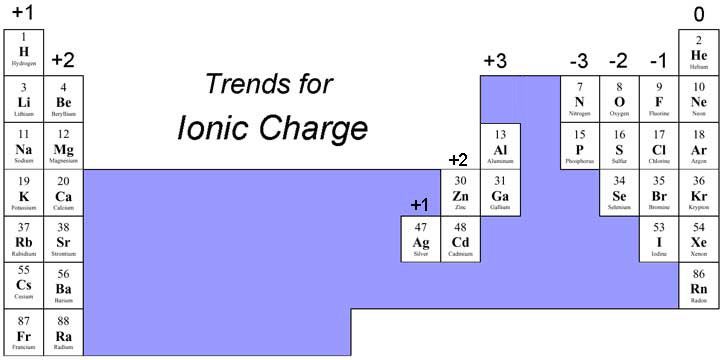
Common Ion Charges

Here are some of the most common ion charges you should be familiar with:
| Element | Common Charge |
|---|---|
| Hydrogen | +1 (H⁺) |
| Lithium | +1 (Li⁺) |
| Chlorine | -1 (Cl⁻) |
| Magnesium | +2 (Mg²⁺) |
| Oxygen | -2 (O²⁻) |
| Iron | +2 (Fe²⁺), +3 (Fe³⁺) |
Worksheet Guide to Mastering Ion Charges

Utilizing worksheets can significantly enhance your grasp of ion charges. Here’s how to approach them:
Practical Worksheet Exercises

- Element Identification: Given the periodic table, identify the common charges of elements within each group.
- Ion Formation: For each element, write out the ion it forms and its charge.
💡 Note: Pay special attention to transition metals which can form ions with multiple charges.
- Compound Charges: Given a compound, determine the charges of the constituent ions to ensure the compound is neutral.
Tips for Using Worksheets Effectively

- Start Simple: Begin with common monatomic ions before advancing to polyatomic or complex compounds.
- Pattern Recognition: Look for patterns in ion charges, especially within groups of the periodic table.
- Practice Regularly: Consistency is key. Regular practice helps in retaining and understanding ion charge patterns.
- Check Your Work: Use reference materials or ask peers to verify your ion charge assignments.
Worksheet Example
Let’s work through an example worksheet:
| Element | Ion Formed | Charge |
|---|---|---|
| Aluminum | Al³⁺ | +3 |
| Nitrogen | N³⁻ | -3 |
| Bromine | Br⁻ | -1 |
💡 Note: Transition metals often form ions with variable charges; ensure you’re aware of the context in which they are used.
Summing It All Up

The journey through mastering ion charges is both enlightening and essential for chemistry enthusiasts. By understanding why ion charges matter, how to identify and work with ions, and by utilizing practical exercises, you’ve laid the groundwork for a deeper comprehension of chemical interactions. Remember, the patterns in ion charges, the significance in bonding, and the reactivity insights will prove invaluable in your chemical studies and beyond. Keep practicing with worksheets, and you’ll find that ion charges become second nature in no time.
What are the most common charges for ions?

+
The most common ion charges include +1, -1, +2, -2, and -3. Elements like hydrogen and alkali metals typically form +1 ions, halogens form -1 ions, and alkaline earth metals form +2 ions. Elements like oxygen usually take a -2 charge when forming oxides.
Why do some metals form ions with multiple charges?

+
Transition metals, located in the d-block of the periodic table, often form multiple ions because they have d-orbitals available to lose or accept electrons, which can lead to variable oxidation states.
How can ion charges affect the properties of compounds?

+
Ion charges influence properties like solubility, conductivity in solution, melting and boiling points, and the reactivity of compounds. For example, compounds with strong ionic bonds due to high charge differences tend to have higher melting points.



