5 Redox Reactions Tips for Chapter 20 Worksheet

Redox reactions are fundamental to understanding the chemistry behind many natural and industrial processes. They involve the transfer of electrons between species, leading to oxidation and reduction. As you tackle Chapter 20 in your chemistry curriculum, mastering redox reactions is crucial for achieving academic success. Here are five tips to help you excel with your redox reaction worksheet:
1. Understand the Basics

Before diving into complex redox reactions, ensure you understand the foundational concepts:
- Oxidation: The loss of electrons from an atom or ion.
- Reduction: The gain of electrons by an atom or ion.
- Oxidation States: Also known as oxidation numbers, these help track the movement of electrons.
Recognize that in any redox reaction, the number of electrons lost must equal the number of electrons gained. This principle of electron balance is key to solving redox problems.
2. Practice Identifying Redox Reactions

Identifying redox reactions is the first step in solving problems. Here’s how:
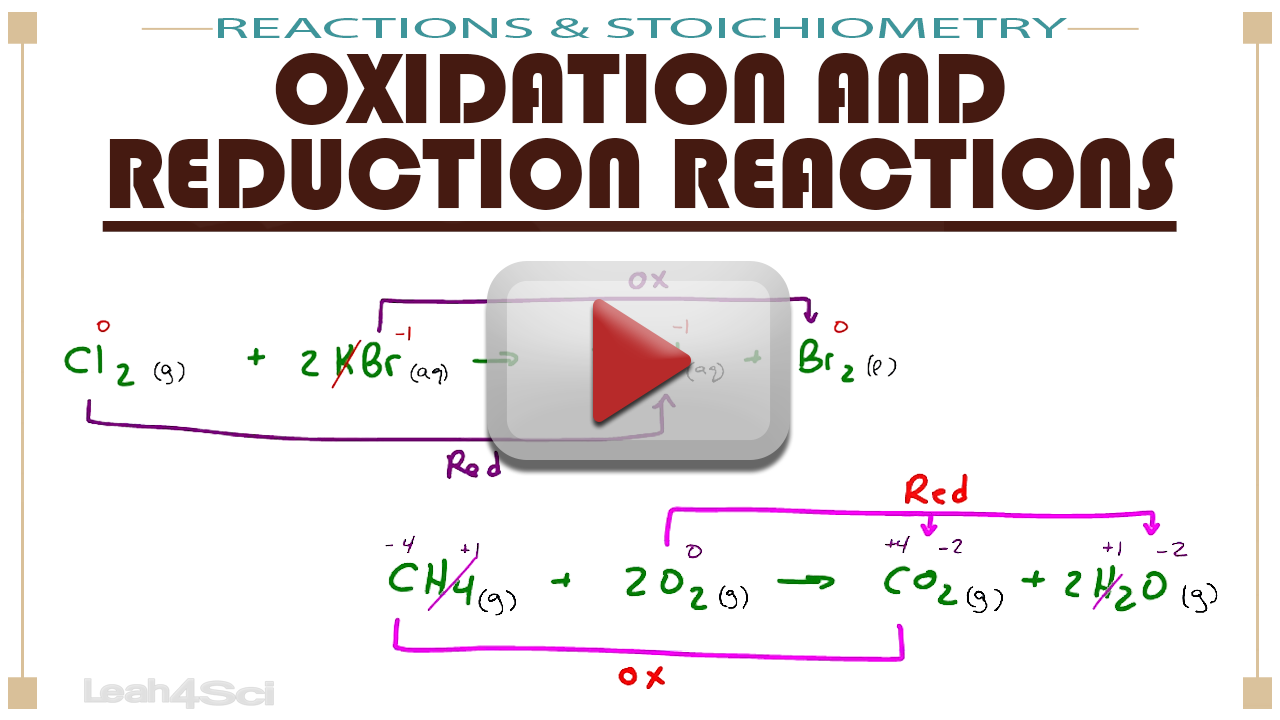
- Look for changes in oxidation states. If an element changes from one oxidation state to another, it’s a redox reaction.
- Use the mnemonic OIL RIG (Oxidation Is Loss, Reduction Is Gain) to remember electron transfer.
Use examples from everyday life, like rusting (oxidation of iron) or combustion reactions, to familiarize yourself with redox reactions in a practical context.
3. Master Balancing Redox Equations

Balancing redox equations can be challenging but essential. Here are two methods:
- The Half-Reaction Method: Break the reaction into two half-reactions (one for oxidation and one for reduction). Balance atoms other than hydrogen and oxygen first, then balance oxygen using water, followed by hydrogen using hydrogen ions. Balance charge by adding electrons, and finally, make electron transfer equal in both half-reactions.
- The Ion-Electron Method: Similar to the half-reaction method but often used for more complex reactions in acidic or basic conditions.
| Step | Action |
|---|---|
| 1 | Write the skeleton equation |
| 2 | Identify oxidation and reduction |
| 3 | Balance non-oxygen and hydrogen atoms |
| 4 | Balance oxygen by adding H2O |
| 5 | Balance hydrogen by adding H+ |
| 6 | Balance charge by adding electrons |
| 7 | Equalize the number of electrons |
| 8 | Combine the half-reactions |
| 9 | Adjust in basic conditions if necessary |

💡 Note: It's beneficial to practice both methods to understand which suits you best for different types of reactions.
4. Utilize Redox Table for Standard Potentials

Understanding the standard electrode potentials can help predict the direction of redox reactions:
- The electrode potential provides the voltage of each half-cell relative to the standard hydrogen electrode (SHE).
- Use the table to find out which species will oxidize or reduce based on their standard reduction potential (E°).
Keep in mind that a higher reduction potential means the species is more likely to be reduced:
- If E° is positive, the reaction is spontaneous.
- If E° is negative, the reaction is non-spontaneous and requires an input of energy.
🔋 Note: Don't forget that for oxidation, you'll need to reverse the sign of the reduction potential to calculate the cell potential.
5. Use Redox Reactions to Solve Electrochemistry Problems

Redox reactions are integral to electrochemistry, which includes:
- Galvanic Cells: Spontaneous redox reactions that produce electrical energy.
- Electrolytic Cells: Non-spontaneous reactions where electrical energy drives the redox reactions.
Here are some steps to tackle electrochemistry problems:
- Determine the half-reactions involved.
- Calculate the standard cell potential (E°cell) using the difference in standard reduction potentials.
- Use Faraday's laws to calculate mass change, charge, or current in an electrolytic cell.
In conclusion, redox reactions are more than just chemical equations; they are the driving force behind many of the processes that shape our environment and technology. By focusing on these five key areas, you can improve your understanding of redox reactions, making your chapter 20 worksheet and related exam questions seem less daunting. Remember, practice makes perfect, and real-world examples can provide insight into how these reactions work in nature and industry. Happy learning!
What are the common signs of a redox reaction?
+
Common signs include changes in oxidation states, color changes, generation of gases, formation of precipitates, or energy changes (exothermic or endothermic reactions).
Why is it important to balance redox equations?

+
Balancing redox equations ensures the law of conservation of mass is maintained, as atoms cannot appear or disappear in a chemical reaction. It also allows for accurate calculation of the quantity of substances involved.
How do I differentiate between galvanic and electrolytic cells?

+
Galvanic cells produce electrical energy from spontaneous redox reactions, while electrolytic cells use electrical energy to drive non-spontaneous redox reactions.