5 Must-Know Facts about Matter and Change

Exploring the fundamental nature of the universe involves a deep dive into the realm of matter and its interaction through change. These two concepts are at the heart of chemistry and physics, revealing the secrets of everything from the smallest particle to the vast cosmos. In this comprehensive guide, we will uncover five must-know facts about matter and change, ensuring that you'll walk away with a richer understanding of how the world works at its core.
The Duality of Matter: Physical vs. Chemical Changes
One of the most basic distinctions in chemistry is between physical and chemical changes:
- Physical Change: A physical change alters the form or appearance of a material without changing its chemical composition. Examples include melting ice into water, boiling water into steam, or grinding a piece of chalk into powder. These changes can often be reversed through simple physical processes.
- Chemical Change: A chemical change, on the other hand, involves a transformation at the molecular level where one or more substances react to produce new substances. These changes are generally irreversible; for example, burning wood produces ashes, carbon dioxide, and water vapor, elements that cannot be simply reassembled into wood.
📌 Note: Not all changes are immediately apparent; sometimes, what seems like a physical change might be an indicator of a chemical one.
Conservation of Mass and Energy

Two fundamental laws that underpin the study of matter and change are:
- Law of Conservation of Mass: This law states that mass cannot be created or destroyed in an isolated system. When a chemical reaction occurs, the total mass of the reactants must equal the total mass of the products. This principle helps to balance chemical equations.
- Law of Conservation of Energy: Energy, like mass, is conserved. During any change, whether physical or chemical, the total energy of a system remains constant, though it can be transferred between different forms (e.g., kinetic, thermal, potential).
The Periodic Table: A Universal Guide to Matter

The Periodic Table is not just a chart but a powerful tool for understanding matter:
- Each element in the table has a unique set of properties, and their position reflects these characteristics. For example, elements within the same group (column) share similar chemical behavior due to having the same number of valence electrons.
- The table organizes elements into categories like metals, non-metals, and metalloids, each with distinct physical and chemical properties, facilitating predictions about their interactions and reactivity.

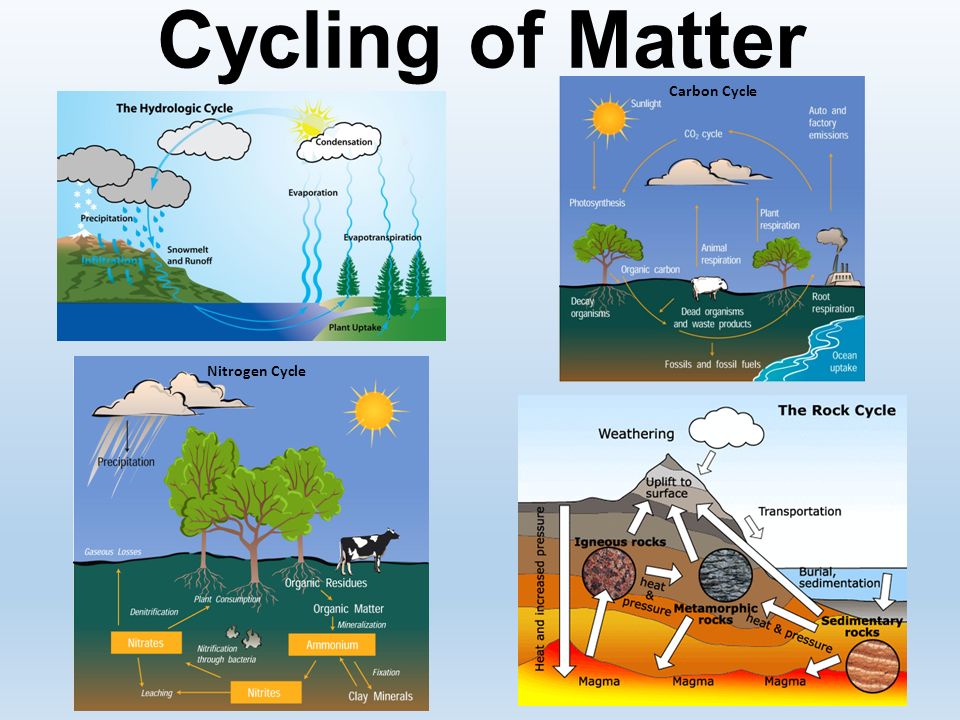
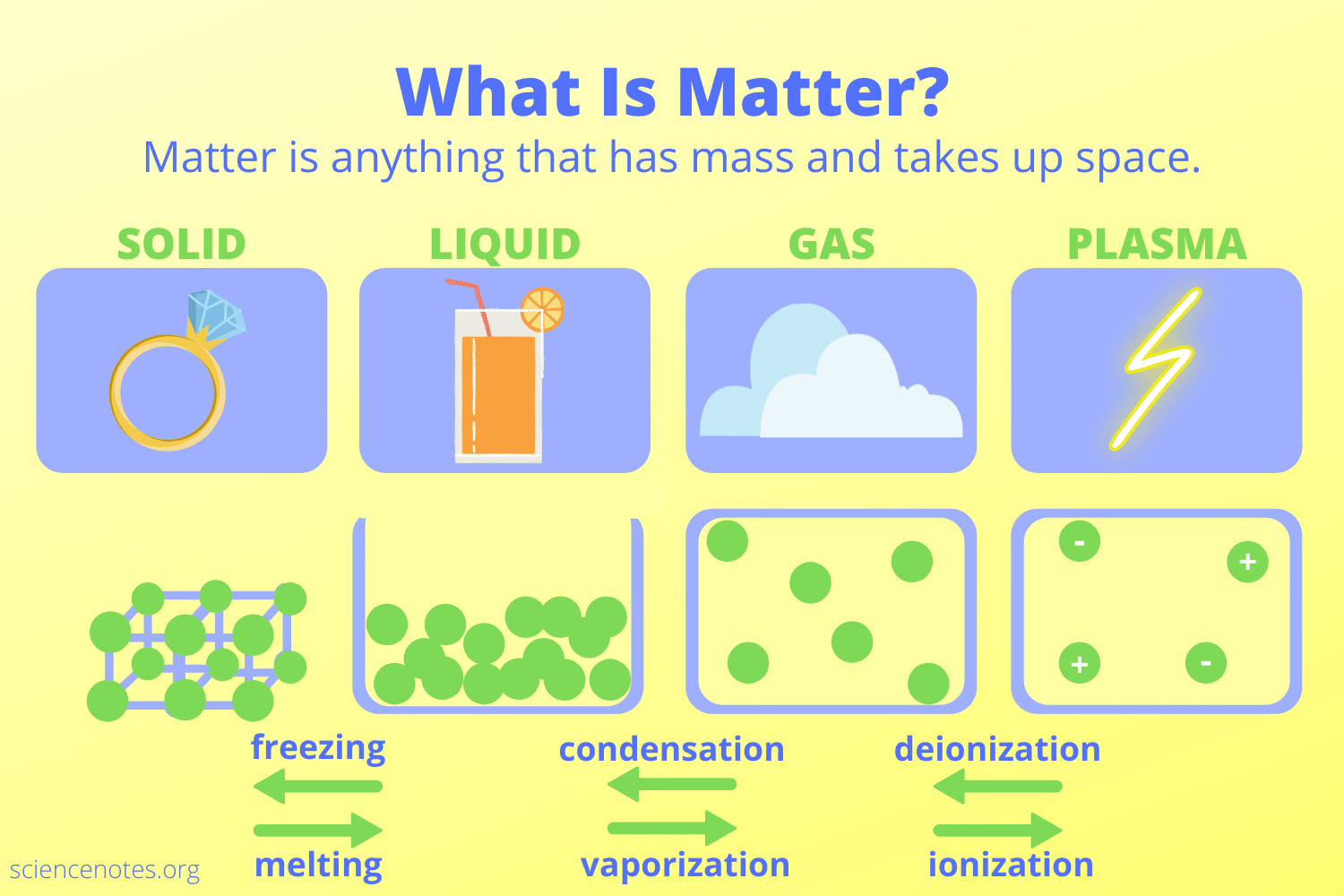
Phases of Matter

Matter can exist in various states or phases, each with distinct characteristics:
- Solid: Particles are closely packed and have a fixed volume and shape. Ice, diamond, and wood are examples.
- Liquid: Here, particles are close but can move past each other, giving liquids a fixed volume but no fixed shape. Water, mercury, and oil illustrate this state.
- Gas: Gases have particles far apart with no fixed volume or shape, filling any container they occupy. Oxygen, helium, and carbon dioxide are common gases.
- Plasma: The fourth state of matter, where ions and electrons coexist in a cloud-like state, often seen in stars or lightning.
- Other Phases: There are also less common states like Bose-Einstein condensates and superfluids, observed at extreme conditions.
| State of Matter | Volume | Shape | Particle Movement |
|---|---|---|---|
| Solid | Fixed | Fixed | Vibrational |
| Liquid | Fixed | Variable | Flowing |
| Gas | Variable | Variable | Random, free motion |
| Plasma | Variable | Variable | Electrified, ions and electrons move freely |
Energy Changes and Chemical Bonds
The formation or breaking of chemical bonds involves energy:
- Endothermic Reactions: These reactions absorb heat energy from their surroundings, causing a decrease in temperature. The energy is often used to break bonds, like the melting of an ice cube in water.
- Exothermic Reactions: Here, energy is released, usually in the form of heat or light. Combustion, like the burning of a candle, where new bonds form, is an example of an exothermic reaction.
In conclusion, our journey through the facts of matter and change has illuminated how these concepts dictate the behavior of everything in our universe. Understanding the distinctions between physical and chemical changes, the conservation laws of mass and energy, the utility of the Periodic Table, the different phases of matter, and the significance of energy in chemical processes equips us to better comprehend the natural world, from the microscopic to the macroscopic level. Remember, science is a journey of discovery where every fact learned opens new doors to further understanding.
What is the difference between a chemical and a physical change?

+
A chemical change produces new substances, changing the matter at a molecular level. A physical change only alters the appearance or state of matter without creating new compounds.
Why does the conservation of mass and energy matter?
+
These laws ensure that no matter or energy is lost during a reaction, allowing scientists to predict outcomes, balance equations, and understand natural processes.
Can all elements change phases?

+
Most elements can exist in multiple phases under different temperature and pressure conditions. However, some elements like helium only exist as gas or solid under standard conditions.



