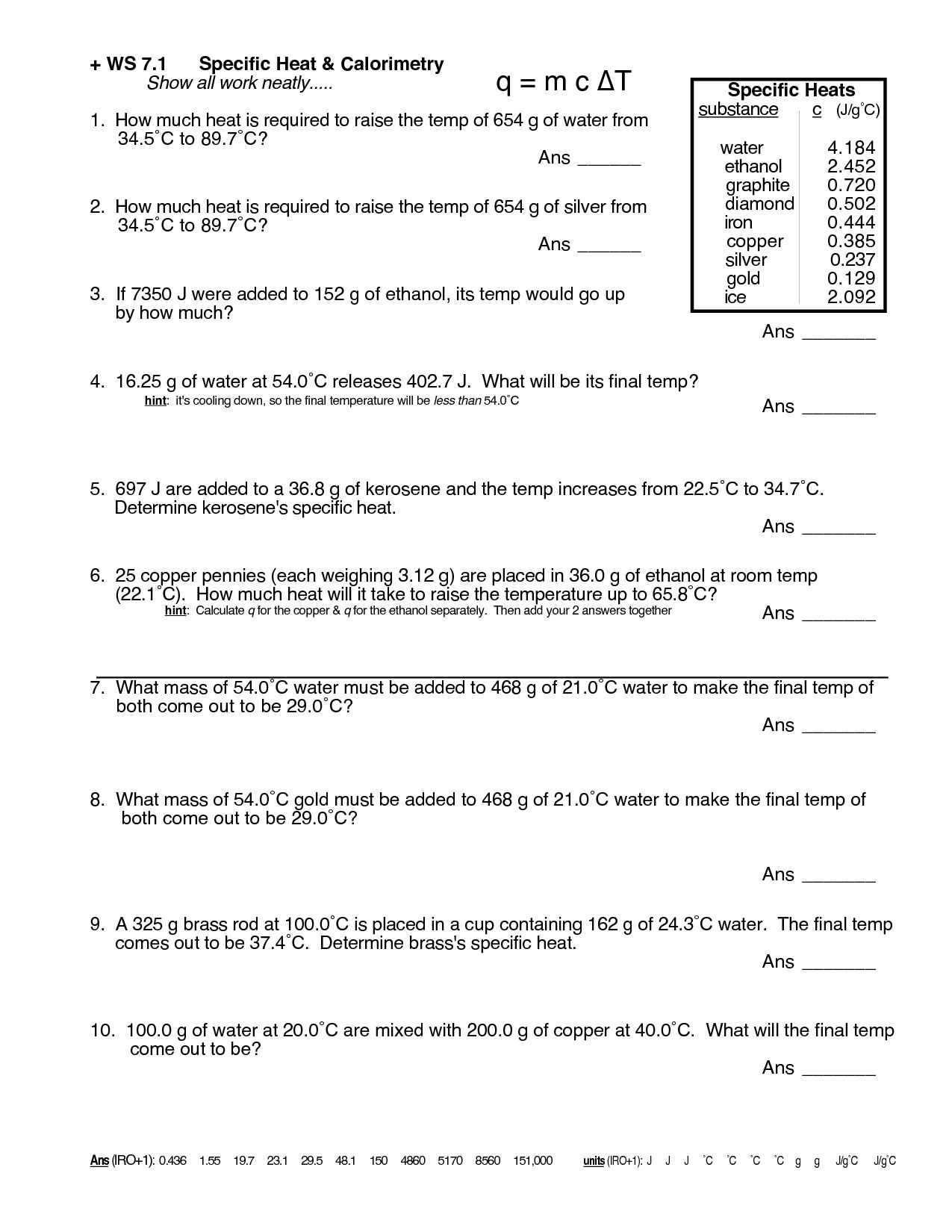
Master Specific Heat Calculation with This Worksheet

Welcome to a comprehensive guide on mastering the calculation of specific heat with the aid of a tailored worksheet. Specific heat is a fundamental concept in thermodynamics, impacting industries ranging from food processing to aerospace engineering. Understanding and accurately calculating specific heat can unlock insights into material behavior under different thermal conditions, making it an essential skill for scientists, engineers, and enthusiasts alike. In this blog, we'll dive into the specifics of how specific heat is calculated, why it matters, and how you can improve your understanding through practical application with a worksheet.
What is Specific Heat?

Specific heat, denoted as c or Cp, is the amount of heat per unit mass required to raise the temperature by one degree Celsius (or one Kelvin). This property varies between materials due to differences in their molecular structure, density, and other physical properties. Here’s how specific heat is defined:
- Formula: q = mcΔT, where:
- q is the heat added
- m is the mass of the substance
- c is the specific heat capacity
- ΔT is the change in temperature
🌡 Note: The specific heat capacity is different from molar heat capacity, which considers the amount of heat per mole of the substance.
Why Specific Heat Matters

The importance of specific heat spans across various applications:
- Heat Transfer: It dictates how quickly or slowly a substance can be heated or cooled, affecting energy efficiency in systems.
- Material Selection: In engineering, choosing materials with the right specific heat can optimize performance in components subject to thermal cycles.
- Energy Storage: Materials with high specific heat capacity are used for thermal energy storage systems, like solar power plants.
- Cooking and Food Industry: Understanding specific heat helps in predicting cooking times and preserving nutritional value by controlling temperature changes.
Calculating Specific Heat: Step-by-Step Guide

Collect Data

- Mass of the sample (m)
- Initial temperature (Ti)
- Final temperature (Tf)
- Heat supplied or absorbed (q)
Apply the Formula

Use the formula c = q / (m * ΔT), where:
- ΔT is Tf - Ti
Example Calculation

| Parameter | Value |
|---|---|
| Mass of water (kg) | 2.0 |
| Initial Temperature (°C) | 20 |
| Final Temperature (°C) | 45 |
| Heat supplied (kJ) | 200 |
Let's calculate:
- ΔT = 45 - 20 = 25°C
- q = 200 kJ
- m = 2 kg
- c = 200 kJ / (2 kg * 25°C) = 4 kJ/(kg°C)
🌟 Note: This example uses water, which has a well-known specific heat capacity of approximately 4.186 kJ/(kg°C) at room temperature.
Practical Worksheet for Specific Heat Calculation

A worksheet can turn theoretical knowledge into practical skills. Here’s how to use one effectively:
Worksheet Structure

Your worksheet should include:
- Section for listing parameters
- Space for calculations
- Examples or problems for practice
Example Problems

Here are some exercises you might include:
- Calculate the specific heat of a 500-gram metal sample heated from 25°C to 75°C, absorbing 15 kJ of heat.
- If a material has a specific heat of 0.45 kJ/kg°C, how much heat is required to raise 3 kg of this material by 50°C?
✅ Note: Practicing with a variety of materials and conditions helps in understanding the real-world implications of specific heat capacity.
Incorporating Specific Heat into Engineering Designs

Here are some practical applications where understanding specific heat is crucial:
Thermal Energy Storage

- Select materials like molten salts or phase change materials for solar power plants to store heat efficiently.
Cooling Systems

- Engineers design cooling systems considering the specific heat capacity of coolants to ensure effective heat dissipation.
Insulation

- Choose insulation materials that have low thermal conductivity and appropriate specific heat to minimize energy losses.
Summary

Throughout this blog, we've explored the concept of specific heat capacity, its significance in various applications, and practical methods for its calculation. By understanding the amount of heat energy required to change the temperature of different substances, we can make informed decisions in fields ranging from food preservation to energy efficiency in buildings. Whether you're an engineer, a scientist, or simply someone curious about thermodynamics, mastering the calculation of specific heat opens up a world of innovation and efficiency. Remember, practice is key, and a well-designed worksheet can be an excellent tool for sharpening your skills in this area.
What is the difference between specific heat and heat capacity?

+
Specific heat refers to the amount of heat per unit mass required to raise the temperature of a substance by one degree Celsius. Heat capacity, on the other hand, is the amount of heat needed to raise the temperature of the entire substance by one degree, considering its total mass.
Why does specific heat vary with temperature?

+
Specific heat can change with temperature due to alterations in the material’s molecular structure, phase changes, or thermal expansion. At low temperatures, the atomic vibrations increase linearly with temperature, leading to higher specific heat values.
How can I measure specific heat in a lab?
+You can measure specific heat by using methods like calorimetry. Here’s a basic approach:
- Measure the mass of a sample.
- Heat the sample to a known temperature.
- Transfer the sample to a calorimeter containing a known mass of water at a known temperature.
- Measure the equilibrium temperature of the system after thermal equilibrium is reached.
- Use the heat transfer equations to calculate the specific heat of the sample.
What materials have high specific heat?
+Materials like water, certain oils, and salts used in thermal storage have high specific heat capacities, making them ideal for applications requiring substantial heat absorption or release.
Can specific heat affect everyday life?
+Absolutely. The specific heat of materials in our homes, like the insulation in our walls or the cooking pots we use, influences how quickly our living spaces warm up or cool down, how food is cooked, and how we conserve energy.