Master Bronsted Lowry Acids & Bases with Our Worksheet

The principles of Bronsted Lowry acids and bases are fundamental in chemistry, offering a broad perspective on acid-base reactions. These principles not only deepen our understanding of chemical behaviors but also have practical applications in various fields, including environmental science, medicine, and engineering. This guide will walk you through the intricacies of Bronsted Lowry acids and bases, using an engaging worksheet to help reinforce your learning.
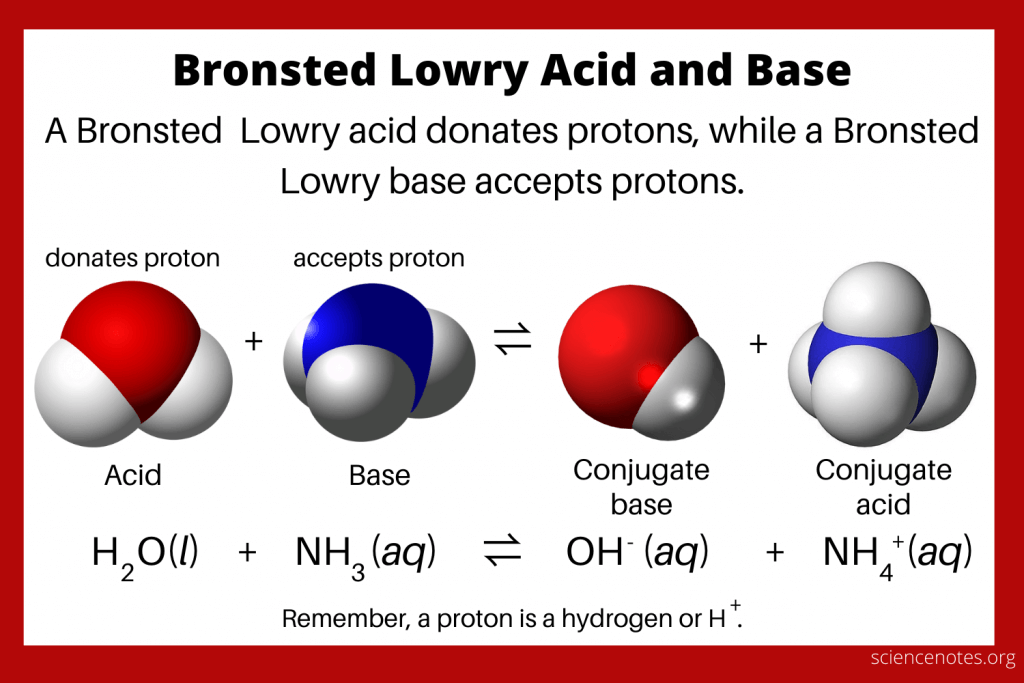
What are Bronsted Lowry Acids and Bases?

The Bronsted Lowry theory expands upon the traditional Arrhenius model of acids and bases. Here’s how it works:
- Acid: A substance that donates a proton (H+) in a chemical reaction.
- Base: A substance that accepts a proton (H+) in a chemical reaction.
Working Through the Worksheet

To master the concepts, let’s dive into the worksheet:
1. Identification

Identify the acid and base in each given reaction. Here’s an example:
| Reaction | Bronsted Lowry Acid | Bronsted Lowry Base |
|---|---|---|
| HCl + NH3 → NH4+ + Cl- | HCl | NH3 |

This reaction shows that HCl is the proton donor (acid), and NH3 is the proton acceptor (base).
2. Conjugate Pairs

In the example above, after the reaction:
- HCl → Cl- + H+ (Conjugate base pair)
- NH3 + H+ → NH4+ (Conjugate acid pair)
💡 Note: Conjugate pairs are formed when an acid loses a proton or a base gains one.
3. pH Calculations
Use the following steps to calculate the pH of a Bronsted Lowry acid:
- Identify the concentration of the acid.
- Find the pKa value (negative logarithm of the acid dissociation constant).
- Use the Henderson-Hasselbalch equation for weak acids:
pH = pKa + log([A-]/[HA])
4. Buffer Solutions

Understand how buffers resist changes in pH:
- Buffer solutions contain a weak acid and its conjugate base.
- They maintain pH by consuming additional acid or base.
Key Takeaways

Through this worksheet, we’ve explored the mechanics of Bronsted Lowry acids and bases, how to identify them, and their interactions in various reactions. Here are some key points to remember:
- The Bronsted Lowry definition is broader and more inclusive than the Arrhenius definition.
- Conjugate acid-base pairs are crucial for understanding equilibrium in reactions.
- The ability to calculate pH and understand buffering capacity is essential for many practical applications.
As you progress in your chemistry studies, keep applying these principles. The knowledge of Bronsted Lowry acids and bases will enhance your ability to predict reactions, control pH in solutions, and manipulate chemical processes effectively.
What is the difference between Bronsted Lowry and Arrhenius definitions of acids and bases?

+
The Arrhenius definition states that acids produce H+ ions and bases produce OH- ions in aqueous solution. In contrast, the Bronsted Lowry theory defines acids as proton donors and bases as proton acceptors, which applies in both aqueous and non-aqueous environments.
How can I identify Bronsted Lowry acids and bases?
+
Look for molecules or ions that can donate or accept protons. In reactions, Bronsted Lowry acids will have an H+ that can be donated, and bases will have an atom with lone pairs of electrons to accept a proton.
Why is pH important in acid-base reactions?

+
pH directly affects the rate and extent of many chemical reactions. It influences the solubility of salts, the enzymatic activity, and the behavior of pharmaceuticals, making it crucial for understanding and controlling these processes.