Boyle's Law Worksheet Answers: Master Gas Laws Easily

Understanding Boyle's Law and Its Applications

Boyle’s Law is a fundamental principle in the study of gases and plays a critical role in both theoretical physics and practical applications. This law, stated by Robert Boyle in the 17th century, explains how the pressure of a gas changes with its volume when the temperature and the amount of gas remain constant. Here, we’ll delve into Boyle’s Law, its implications, practical applications, and how you can use it to solve problems effectively.
What is Boyle's Law?
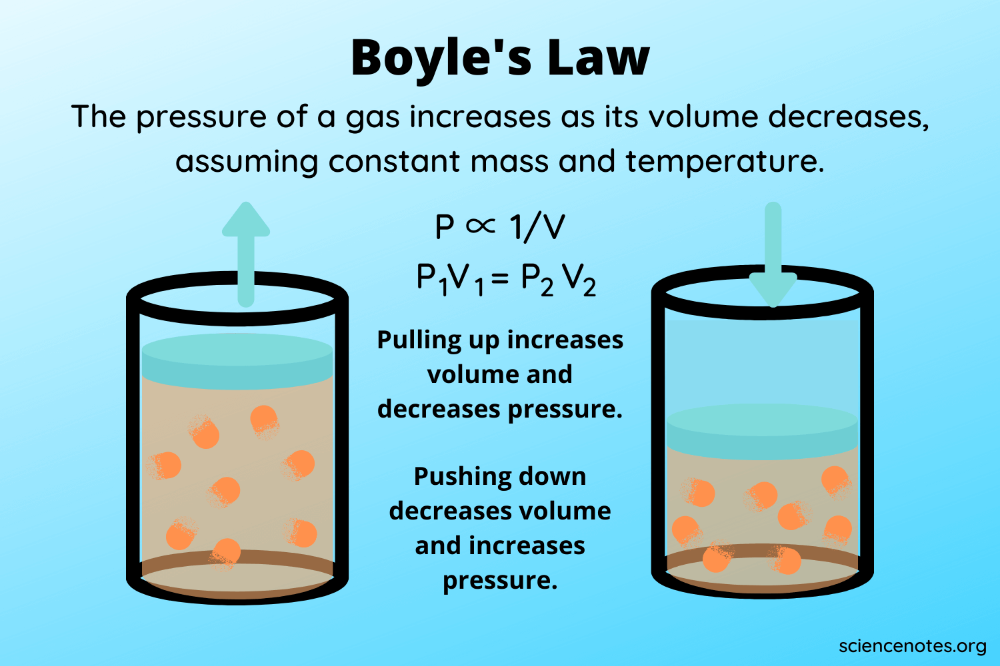
Boyle's Law asserts that the pressure ( P ) of a given quantity of gas varies inversely with its volume ( V ) at a constant temperature. Mathematically, it is expressed as:
[ P_1 \times V_1 = P_2 \times V_2 ]
- P_1 and V_1 are the initial pressure and volume.
- P_2 and V_2 are the final pressure and volume.
This relationship means that if the volume of a gas decreases, its pressure will increase proportionally, and vice versa, as long as the temperature does not change.
Why Boyle's Law Matters
Boyle's Law provides insight into the behavior of gases under different conditions, which is crucial for:
- Designing Air Conditioning and Ventilation Systems: Engineers use Boyle's Law to design systems where air needs to be compressed or expanded.
- Medical Applications: Understanding how gases behave when a patient's lungs expand or contract is essential in respiratory medicine.
- Aeronautics: Gas behavior in flight engines and fuel systems adheres to Boyle's Law.
- Diving Physics: Scuba divers rely on the principles of Boyle's Law to understand how pressure changes affect air volume in their bodies.
Solving Boyle's Law Problems

Here's how to solve typical Boyle's Law problems:
- Identify the given values: You'll need two of the four variables ( P_1, V_1, P_2, V_2 ).
- Substitute the known values into the formula: Plug in the known values into P_1 \times V_1 = P_2 \times V_2 .
- Solve for the unknown variable: Using basic algebra, isolate the unknown value on one side of the equation.
Example: A gas has a volume of 2 liters at a pressure of 3 atmospheres. If the volume is reduced to 1 liter, what will be the new pressure?
[ P_1 = 3 \text{ atm}, V_1 = 2 \text{ L}, V_2 = 1 \text{ L} ] [ P_2 = \frac{P_1 \times V_1}{V_2} ] [ P_2 = \frac{3 \times 2}{1} ] [ P_2 = 6 \text{ atm} ]
⚠️ Note: Always ensure units are consistent when solving gas law problems.
Applications in Everyday Life

Besides its scientific implications, Boyle's Law has several practical applications:
- Scuba Diving: Understanding how pressure affects the volume of gas in diving equipment ensures safe diving practices.
- Air Travel: Cabin pressure adjustments during flight are based on Boyle's Law to maintain a comfortable environment.
- Spray Canisters: The pressure inside an aerosol can decreases as the gas inside expands, following Boyle's Law.
Practical Tips for Learning Boyle's Law

- Use Real-World Examples: Relate Boyle's Law to everyday scenarios like inflating tires or using a syringe to help grasp its concepts.
- Practice with Variety: Solve problems using different units to become comfortable with unit conversion.
- Visualize: Use graphs or sketches to see how pressure and volume change relative to each other.
- Interactive Simulations: Utilize online or software simulations to visually manipulate gas systems and observe Boyle's Law in action.
In sum, Boyle's Law is not just a theoretical equation but a principle with broad implications in our daily lives and technological applications. Whether you are studying for an exam, engaging in scientific research, or simply curious about the world around you, understanding Boyle's Law can provide a deeper appreciation of how gases behave under different conditions. This knowledge equips individuals to address real-world problems with confidence and clarity.
What is Boyle’s Law?

+
Boyle’s Law states that the pressure of a given mass of gas varies inversely with its volume at a constant temperature. It is represented by the formula ( P_1 \times V_1 = P_2 \times V_2 ).
How is Boyle’s Law used in daily life?

+
Boyle’s Law has numerous applications in daily life, such as in scuba diving (buoyancy control), medicine (respiratory therapy), and in the design of air conditioning systems.
Can temperature change affect Boyle’s Law?

+
While Boyle’s Law assumes constant temperature, changes in temperature will invalidate the law unless they are accounted for with other gas laws like Charles’s or Gay-Lussac’s.