Boyle's Law Worksheet: Master Gas Laws Easily

Understanding the behavior of gases is fundamental in both chemistry and physics. Among the various laws that govern these behaviors, Boyle's Law stands out due to its simplicity and applicability. Boyle's Law describes how pressure and volume of a gas relate at constant temperature, and mastering this law can provide you with a deeper insight into gas dynamics. This comprehensive guide will walk you through everything you need to know about Boyle's Law, complete with examples, practice questions, and important notes for better comprehension.
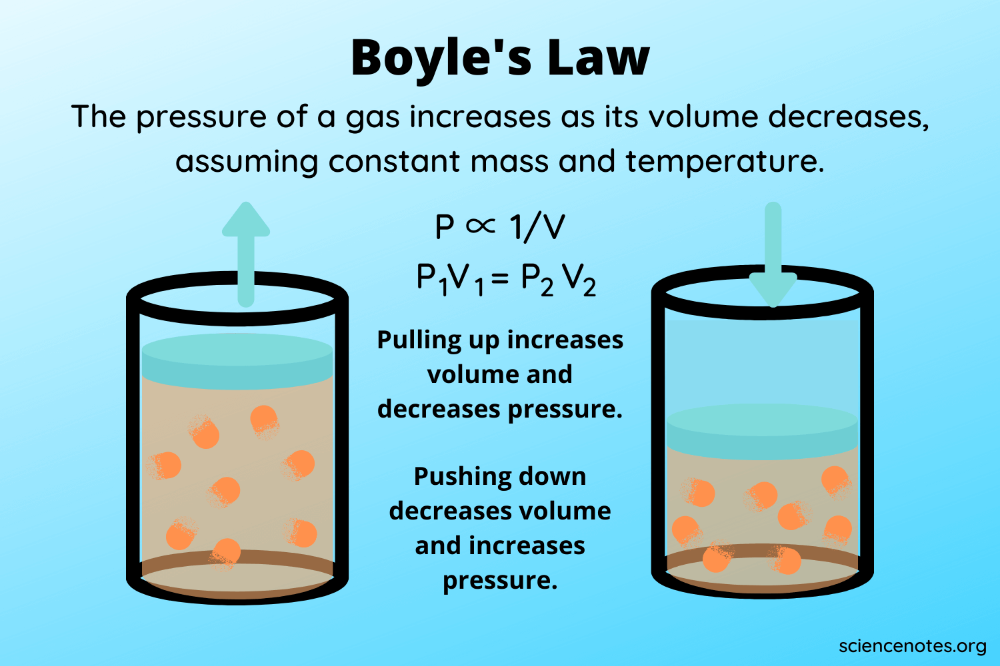
Understanding Boyle’s Law

Boyle’s Law, discovered by Robert Boyle in the 17th century, states that at a constant temperature, the pressure (P) of a given mass of gas is inversely proportional to its volume (V). Mathematically, this relationship can be expressed as:
[ P_1 \times V_1 = P_2 \times V_2 ]
Where:
- P1 and V1 are the initial pressure and volume.
- P2 and V2 are the final pressure and volume after a change.
Key Points of Boyle’s Law

- The temperature must remain constant.
- The number of gas molecules must remain constant (no change in mass).
- Pressure changes if volume changes, and vice versa, provided the conditions above are met.
Examples of Boyle’s Law

Let’s go through some practical examples to understand how Boyle’s Law applies in real-life scenarios:
Example 1: Decreasing Volume

If the volume of a gas in a closed container decreases, its pressure will increase. Imagine a bicycle pump with a piston:
| Volume | Pressure |
|---|---|
| 1 L | 2 atm |
| 0.5 L | 4 atm |

Here, when the volume is halved, the pressure doubles, maintaining the product of pressure and volume as constant:
[ 2 \text{ atm} \times 1 \text{ L} = 4 \text{ atm} \times 0.5 \text{ L} ]
🎨 Note: Remember that Boyle's Law is only accurate for ideal gases where intermolecular forces are negligible.
Example 2: Increasing Volume

If you open a bottle of soda, the pressure decreases as the volume increases. Consider a bottle with a specific volume:
| Volume | Pressure |
|---|---|
| 1 L | 3 atm |
| 2 L | 1.5 atm |
[ 3 \text{ atm} \times 1 \text{ L} = 1.5 \text{ atm} \times 2 \text{ L} ]
Practice Problems

Here are some practice problems to help solidify your understanding:
Problem 1

A balloon contains 2 liters of helium gas at a pressure of 5 atm. If you wish to lower the pressure to 2.5 atm, what should the new volume be?
💡 Note: Use Boyle's Law formula, P1V1 = P2V2, to solve this problem.
Problem 2

A gas has a pressure of 3 atm when it occupies a volume of 10 liters. If the volume is reduced to 5 liters, what will be the new pressure?
Wrap-Up
By now, you should have a good grasp on Boyle’s Law, understanding how pressure and volume interact under isothermal conditions. This law not only simplifies the study of gas behavior but also has practical applications in various fields like scuba diving, engineering, and even medical treatments involving gas pressure. Remember that:
- Boyle's Law is a special case of the ideal gas law where temperature and amount of gas are fixed.
- It's essential for applications involving pressure or volume changes in systems where temperature control is critical.
- The relationship between pressure and volume helps in designing equipment, understanding atmospheric conditions, and more.
Keep practicing with different scenarios, and soon you'll be able to apply Boyle's Law effortlessly. Whether you're dealing with physical experiments, industrial applications, or simply trying to understand the principles behind everyday phenomena, Boyle's Law is an invaluable tool in your scientific toolkit.
What is Boyle’s Law and why is it important?

+
Boyle’s Law establishes a relationship between the pressure and volume of a gas at constant temperature. It’s crucial for understanding gas compression and expansion, which has numerous applications in real-world scenarios like scuba diving, pneumatics, and even in medical equipment like ventilators.
Can Boyle’s Law be applied to real gases?

+
While Boyle’s Law primarily applies to ideal gases, it can still be used as an approximation for real gases at moderate temperatures and pressures where intermolecular forces are minimal.
How does temperature affect Boyle’s Law?

+
Boyle’s Law assumes that the temperature remains constant. If temperature changes, other gas laws like Charles’ Law or Gay-Lussac’s Law need to be considered to understand pressure-volume-temperature relationships.
What happens if volume decreases in a gas container?

+
If the volume of a gas container decreases, assuming constant temperature and mass of gas, the pressure will increase according to Boyle’s Law.