5 Essential Tips to Master Boyle's and Charles Laws
Understanding the Foundations: Boyle's and Charles' Laws

In the fascinating world of thermodynamics, understanding how gases behave under different conditions of pressure, volume, and temperature is crucial. Two fundamental principles that govern these behaviors are Boyle’s and Charles’ Laws. These laws not only simplify complex gas dynamics into understandable concepts but also form the basis for many real-world applications in industries like aviation, engineering, and chemistry. Here, we’ll explore five essential tips that will help you master these pivotal gas laws, ensuring a solid grasp of their applications and implications.
1. Familiarize Yourself with the Inverse Relationship in Boyle's Law


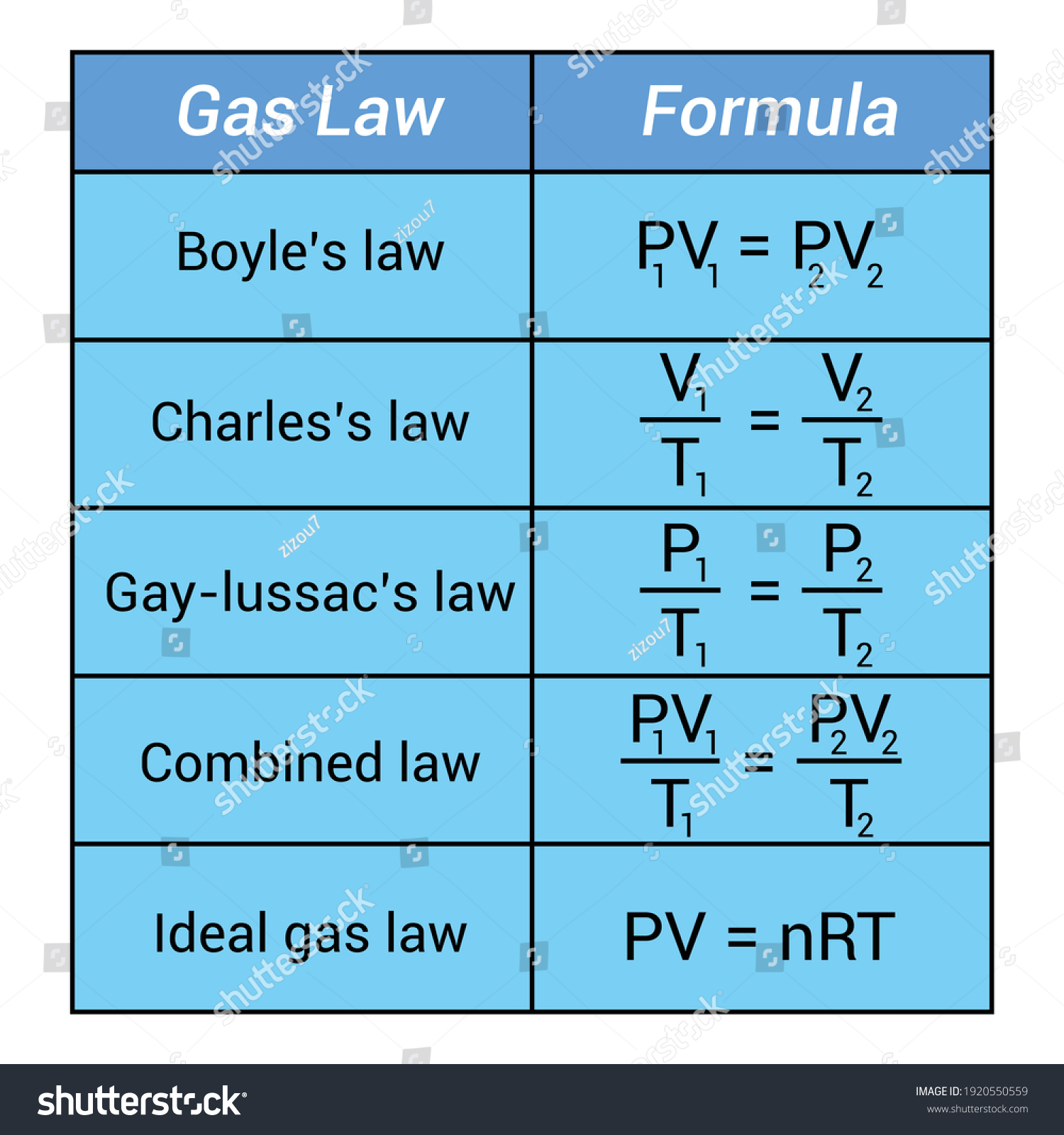
Boyle’s Law, discovered by Robert Boyle in 1662, states that at a constant temperature, the pressure of a gas increases as the volume decreases, and vice versa. This inverse relationship can be expressed mathematically:
P1V1 = P2V2
Where: - P1 and V1 are the initial pressure and volume - P2 and V2 are the final pressure and volume
Tip: To truly understand this law, visualize an inflated balloon. If you squeeze the balloon (decreasing the volume), the pressure inside increases, assuming no air escapes. This simple demonstration helps in grasping the intuitive aspect of Boyle’s Law.
2. Grasp the Direct Proportionality in Charles' Law


Unlike Boyle’s Law, Charles’ Law, formulated by Jacques Charles in 1787, explores the relationship between volume and temperature of a gas at constant pressure. The law can be summarized as:
V1/T1 = V2/T2
Where: - V1 and T1 are the initial volume and absolute temperature - V2 and T2 are the final volume and absolute temperature
Tip: Imagine heating a closed container; as the temperature rises, the gas molecules inside move more quickly, pushing against the walls more often and thus requiring more space. This expansion directly correlates with the temperature increase.
3. Connect Both Laws with the Ideal Gas Equation

To unify Boyle’s and Charles’ Laws, we can use the Ideal Gas Equation:
PV = nRT
Where: - P is the pressure - V is the volume - n is the amount of substance (in moles) - R is the universal gas constant - T is the temperature in Kelvin
Tip: By understanding this equation, you’re not only comprehending the individual laws but also how pressure, volume, temperature, and the amount of gas are interconnected. This holistic approach can deepen your insight into gas behavior.
4. Practical Applications: Making Gas Laws Relatable

Here’s where the rubber meets the road:
Scuba Diving: Understanding Boyle’s Law helps divers manage air consumption and avoid pressure-related injuries like barotrauma. Deeper dives mean higher pressure, which reduces the volume of air in the lungs or scuba gear.
Hot Air Balloons: Charles’ Law explains why hot air balloons rise. As the air inside the balloon is heated, it expands, reducing its density, and thus, the balloon floats due to buoyancy.
| Law | Application | Effect |
|---|---|---|
| Boyle's Law | Scuba Diving | Pressure increases with depth; volume of air in lungs decreases |
| Charles' Law | Hot Air Balloons | Temperature increase causes expansion and reduced density, leading to lift |
5. Real-World Experiments: Hands-On Learning

Experimentation is invaluable in mastering these gas laws:
Boyle’s Law Experiment: Use a syringe and a small ball to demonstrate volume-pressure changes. Seal one end of the syringe with the ball, then compress the plunger. Notice how pressure increases as volume decreases.
Charles’ Law Experiment: Fill a balloon with air and place it in a freezer. Observe how the balloon shrinks due to the decreased temperature, illustrating the law’s principles.
🔍 Note: Use these experiments to solidify your understanding of gas behavior under different conditions.
With these essential tips and practical applications, your journey through Boyle’s and Charles’ Laws will not only become more intuitive but also applicable in everyday scenarios. By linking theoretical knowledge with practical examples, you’ll find that understanding these laws can lead to unexpected insights into how our world works.
Final Thoughts:
Mastering Boyle’s and Charles’ Laws involves more than memorizing equations. It’s about grasping the underlying principles of how gases react to changes in their environment. By visualizing the relationships, applying them to real-world scenarios, and experimenting yourself, these gas laws become more than just scientific rules; they become tools for understanding the fabric of the physical world. Remember, the beauty of these laws lies in their simplicity and the breadth of their applications, from the design of skyscrapers to the travel of gases in space. Whether for educational purposes or for practical applications, these laws are the keys to unlocking the mysteries of gaseous behavior.
What are some common misconceptions about Boyle’s Law?

+
One common misconception is that Boyle’s Law applies universally, regardless of temperature. However, Boyle’s Law is valid only when the temperature remains constant, as temperature changes will affect the pressure-volume relationship.
How does Charles’ Law differ from Boyle’s Law?

+
Charles’ Law deals with the relationship between the volume and temperature of a gas at constant pressure, whereas Boyle’s Law deals with the relationship between pressure and volume at a constant temperature. Essentially, Charles’ Law predicts volume expansion with temperature, while Boyle’s Law predicts volume compression with increased pressure.
Can Boyle’s and Charles’ Laws be combined?

+
Yes, through the Ideal Gas Law (PV = nRT), which combines both laws along with Avogadro’s law to describe how changes in pressure, volume, temperature, and moles of gas are related.