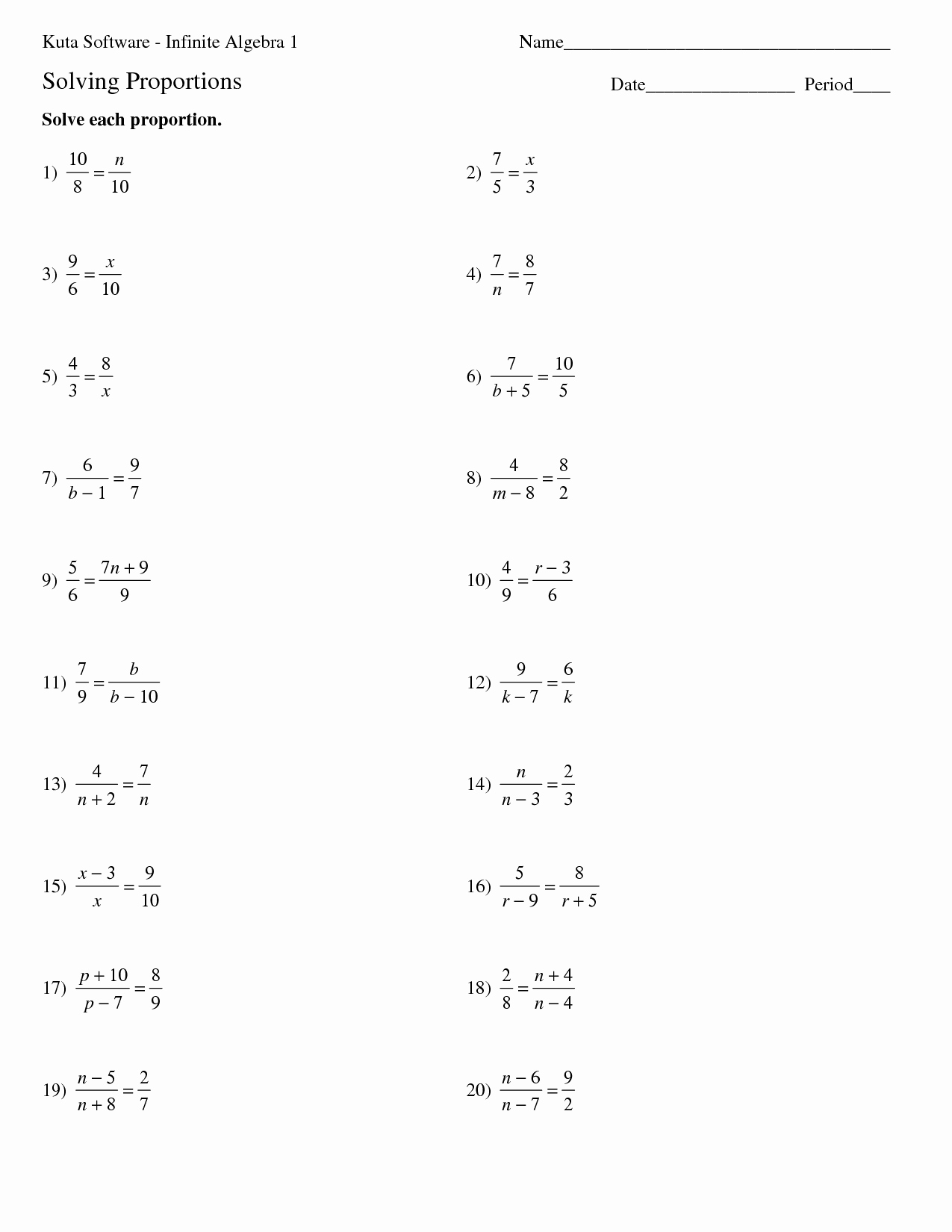Bonding Basics Worksheet Answers Explained Clearly

Understanding how atoms bond to form molecules is foundational in chemistry. This post will delve into the intricacies of bonding basics by explaining the answers to common worksheet questions, providing not just the answers but also the reasoning and context behind them.
The Basics of Atomic Bonds

Atoms combine with each other to form compounds, and how they do this is determined by their electron structure. Here are some key points:
- Valence Electrons: These are the electrons in the outermost shell of an atom, which are involved in bonding.
- Ionic Bonding: Occurs when electrons are transferred from one atom to another, creating ions.
- Covalent Bonding: Involves sharing of electrons between two atoms.
- Metallic Bonding: A bonding where electrons are delocalized and move freely among the metal lattice.
Image: [Bonding Diagram]
Ionic Bonding Explained

Ionic bonding takes place when atoms have a significant difference in their electronegativities. Here’s how it works:
- Electronegative atoms (like chlorine, fluorine) gain electrons.
- Less electronegative or electropositive atoms (like sodium, potassium) lose electrons.
- This exchange forms positive (cations) and negative (anions) ions, which are then electrostatically attracted to each other.
Example: Sodium Chloride (NaCl)
- Na loses an electron, becoming Na+.
- Cl gains this electron, forming Cl-.
- The resulting electrostatic force keeps them bonded in a crystalline structure.
Covalent Bonding

In covalent bonding, atoms share electrons to achieve a stable electron configuration:
- Can be single bonds (one pair of electrons), double bonds (two pairs), or triple bonds (three pairs).
- The bond strength increases with the number of electron pairs shared.
| Compound | Bond Type | Electron Pair | Examples |
|---|---|---|---|
| Water (H2O) | Single Covalent | One | Water, Methane (CH4) |
| Oxygen (O2) | Double Covalent | Two | Oxygen, Carbon Dioxide (CO2) |
| Nitrogen (N2) | Triple Covalent | Three | Nitrogen, Ethyne (C2H2) |

Example: Hydrogen (H2)
- Each hydrogen atom has one valence electron.
- They share this electron to fulfill their 2 electron requirement, forming a stable bond.
Practical Examples of Bonding

Let's look at some everyday compounds to see bonding in action:
Water (H2O)

Water exemplifies covalent bonding:
- Oxygen has six valence electrons, needing two more to complete its octet.
- Two hydrogen atoms, each with one electron, can share their electrons with oxygen.
- This creates two OH single bonds, forming H2O.
Sodium Chloride (NaCl)

In contrast, sodium chloride illustrates ionic bonding:
- Sodium donates one electron, becoming Na+.
- Chlorine accepts this electron, becoming Cl-.
- The resulting ions are attracted to form an ionic lattice.
Understanding Worksheet Answers

Here are some common worksheet questions explained:
Question 1: What type of bond will form between the following elements?

- Na and Cl: Ionic bonding. Sodium (electropositive) will lose an electron to chlorine (electronegative), creating Na+ and Cl-.
- H and O: Covalent bonding. Both hydrogen and oxygen share electrons.
⚠️ Note: Remember, the difference in electronegativity determines the type of bond. A large difference leads to ionic, a small difference to covalent.
Question 2: Describe the electron transfer or sharing in the bond formation of MgCl2.

Magnesium chloride (MgCl2) involves:
- Mg loses two electrons, becoming Mg2+.
- Each Cl gains one electron, becoming Cl-.
- The resulting ions bond via electrostatic forces.
Question 3: How do covalent bonds differ from ionic bonds?

Here are the key differences:
| Feature | Ionic Bonds | Covalent Bonds |
|---|---|---|
| Electron Transfer | Electrons are transferred completely. | Electrons are shared between atoms. |
| Electron Negativity Difference | Large (often > 1.7) | Small to moderate. |
| Forming | Occurs between metals and non-metals. | Typically occurs between non-metals. |
| Bond Strength | Electrostatic forces are strong. | Strength depends on electron sharing. |
| Physical State | Solid crystal lattice, often brittle. | Varies: solid, liquid, or gas. |
In summary, understanding the basics of atomic bonds not only clarifies how elements form compounds but also explains the behavior and properties of those compounds. Ionic bonding involves electron transfer, forming charged ions that are attracted to each other. Covalent bonding involves electron sharing, creating molecules with characteristic strengths and directions of bonds. This knowledge not only helps with worksheet answers but also with conceptual understanding in chemistry.
What is the difference between a cation and an anion?

+
A cation is a positively charged ion that has lost electrons, while an anion is a negatively charged ion that has gained electrons.
How do ionic bonds and covalent bonds affect the physical properties of compounds?

+
Ionic compounds form crystalline structures with high melting and boiling points due to strong electrostatic forces. Covalent compounds often have variable physical states (solid, liquid, or gas) depending on the strength and number of covalent bonds.
Why do covalent bonds sometimes involve the sharing of more than one electron pair?

+
Atoms achieve stable electron configurations by sharing multiple pairs of electrons. This is seen in double bonds (two pairs) or triple bonds (three pairs) where atoms need to gain more electrons to achieve stability or complete an octet.