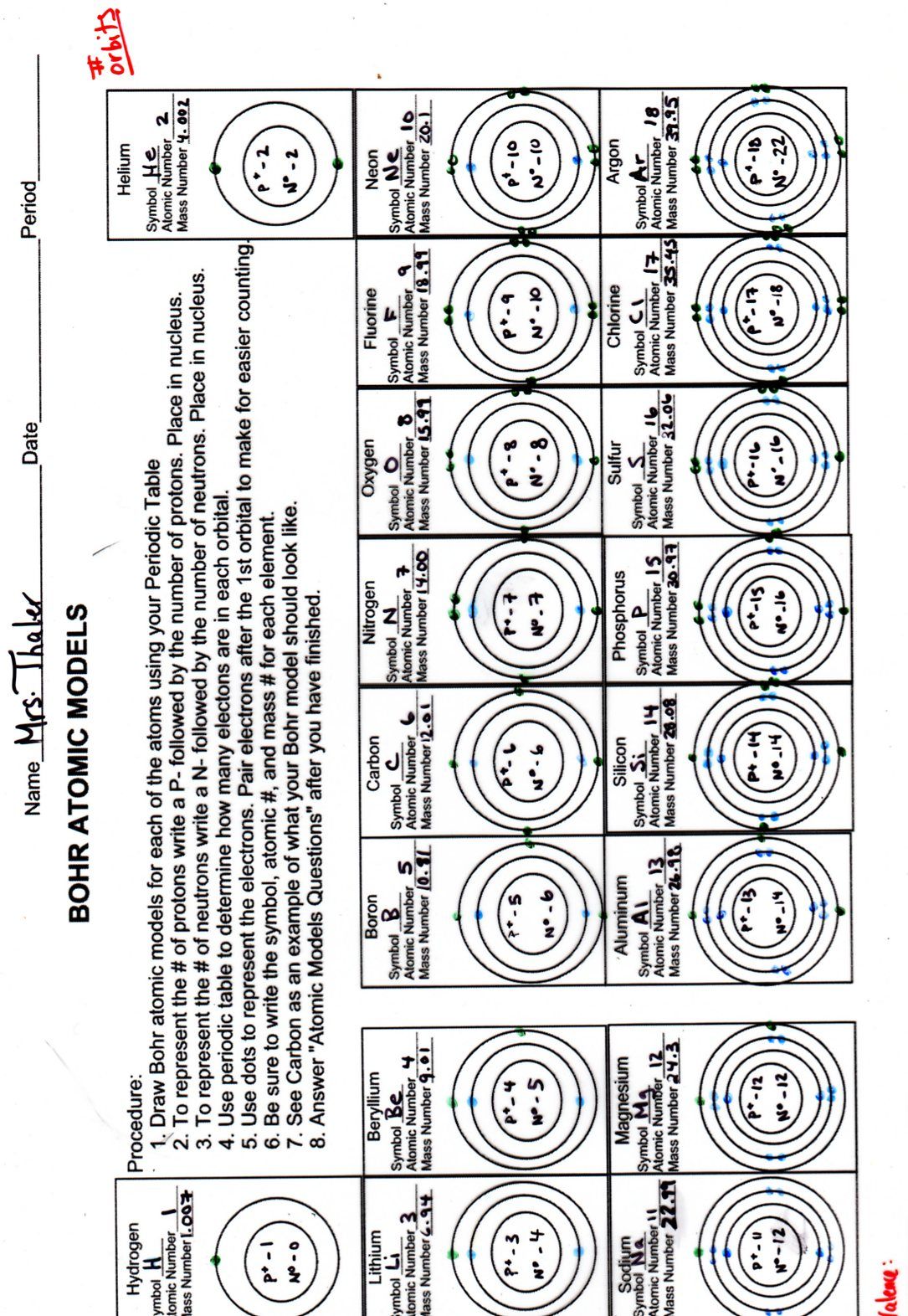
5 Key Answers for Bohr Model Worksheet Mastery

When learning about atomic structure in chemistry, the Bohr model provides a clear visual understanding of how electrons are arranged around the nucleus. For those tackling the Bohr Model Worksheet, achieving mastery requires understanding both its theoretical underpinnings and practical applications. Here are five key answers that can help students excel in their Bohr model assignments:
Understanding Atomic Structure

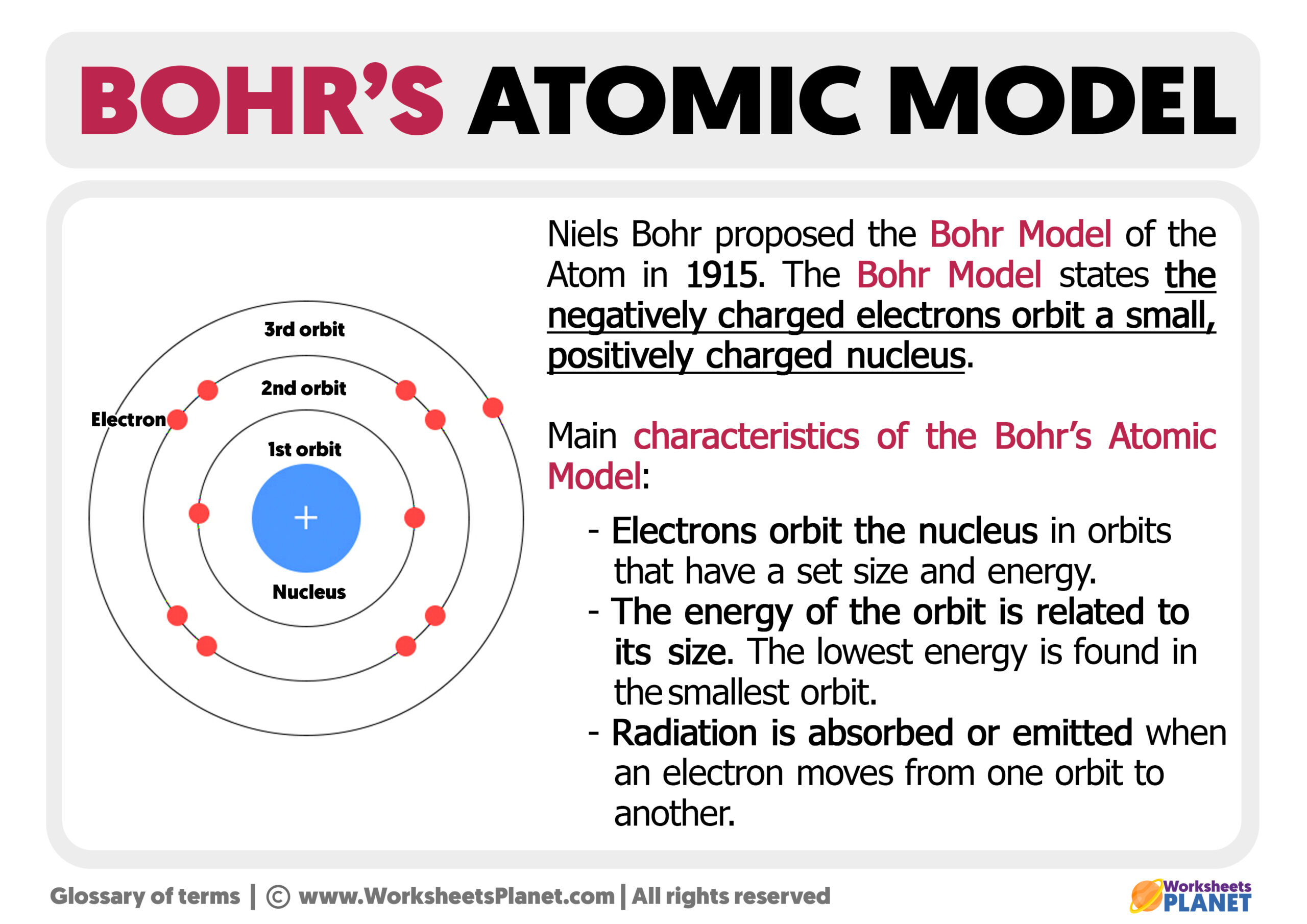
The Bohr model, proposed by Niels Bohr in 1913, visualizes atoms as a small, positively charged nucleus surrounded by electrons that orbit in fixed shells or energy levels. Each shell can hold a specific number of electrons:
- First shell (n=1): up to 2 electrons
- Second shell (n=2): up to 8 electrons
- Third shell (n=3): up to 18 electrons, but the first 10 for most common elements
Key Point: Knowing how many electrons can occupy each shell is crucial when constructing Bohr models.

Determining Electron Configuration
The electron configuration tells you how electrons are distributed among the different energy levels. To find this:
- Identify the element and its atomic number.
- Add electrons to the shells, filling each shell before moving to the next one until all electrons are accounted for.
| Element | Atomic Number | Electron Configuration |
|---|---|---|
| Hydrogen (H) | 1 | 1s1 |
| Helium (He) | 2 | 1s2 |
| Lithium (Li) | 3 | 1s2 2s1 |

Key Point: Use electron configurations to accurately draw Bohr models, ensuring you place electrons in the correct energy levels.
Calculating Energy Levels

In the Bohr model, each orbit (shell) corresponds to a specific energy level. The energy (E) of an electron can be calculated using the formula:
E = -13.6 / n2 eV
Where n is the principal quantum number (the energy level), and eV stands for electron volts.
As electrons move to different energy levels, their energy changes:
- When electrons absorb energy, they jump to a higher energy level.
- When they release energy, they fall back to a lower energy level, emitting photons of light.
⚠️ Note: Understanding energy levels helps explain phenomena like spectral lines.
Predicting Chemical Behavior
The arrangement of electrons around the nucleus as depicted in the Bohr model significantly influences an atom’s chemical behavior:
- Elements with full outer shells (noble gases) are generally inert.
- Elements with incomplete outer shells will interact with other atoms to achieve a stable electron configuration.
By understanding the Bohr model, students can predict:
- Why sodium (Na) readily gives up an electron to become stable.
- Why chlorine (Cl) tends to gain an electron to fill its outermost shell.
Key Point: The outer shell’s electron count determines an element’s chemical reactivity.
Valence Electrons and Electron Dot Structures

Valence electrons, those in the outermost energy level, play a pivotal role in chemical bonding. The Bohr model allows students to:
- Determine the number of valence electrons, which dictates the number of bonds an atom can form.
- Sketch electron dot structures, where each dot represents a valence electron around the atom’s symbol.
This understanding is foundational for learning:
- The octet rule: Atoms tend to combine in such a way that they each have eight electrons in their valence shells, giving them the electron configuration of a noble gas.
- How to draw Lewis structures for molecules.
By mastering this concept, students can explain the structure of simple molecules and understand bonding.

In summary, mastering the Bohr model worksheet involves a deep understanding of atomic structure, electron configurations, energy levels, chemical behavior, and valence electrons. Each of these key answers not only provides a solution to common worksheet problems but also offers a broader insight into the world of chemistry. By focusing on these areas, students can unravel the complexities of atomic theory, visualize chemical interactions, and predict how elements will bond. This foundational knowledge sets the stage for more advanced chemical studies, ensuring a robust understanding of the fundamental principles that govern the matter around us.
Why is the Bohr model important in chemistry?

+
The Bohr model, while simplified, introduced the concept of quantized energy levels for electrons, laying the groundwork for quantum mechanics and the understanding of atomic spectra, which are crucial in modern physics and chemistry.
Can the Bohr model accurately describe all elements?
+
While the Bohr model works well for explaining the spectra of hydrogen and some other light elements, it has limitations for larger atoms where electron-electron interactions become significant, leading to the development of more complex models like the quantum mechanical model.
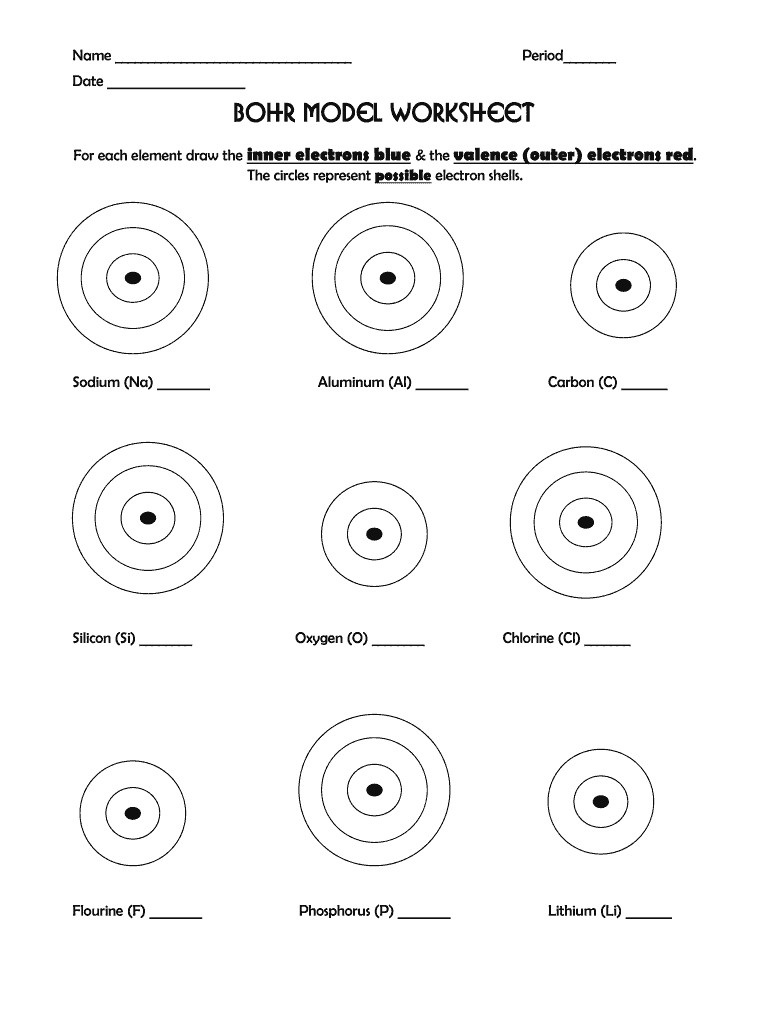
How do I determine the number of shells in a Bohr model?

+
The number of shells in a Bohr model can be estimated by the period number of an element in the periodic table. For example, lithium (Li), which is in period 2, has two shells in its Bohr model.
What is the difference between the Bohr model and the quantum mechanical model?

+
The Bohr model describes electrons as orbiting the nucleus in fixed, circular paths like planets around the sun. In contrast, the quantum mechanical model uses probability and electron cloud concepts, depicting electrons as having probability distributions around the nucleus rather than fixed orbits.
Can electrons jump energy levels in the Bohr model?

+
Yes, electrons can jump between energy levels by absorbing or emitting photons of light. When an electron absorbs energy, it moves to a higher energy level, and when it releases energy, it falls back to a lower level, emitting light at specific wavelengths.



