5 Essential Tips for Bohr Model Worksheet Answers

Understanding the Bohr model is fundamental for students learning about atomic structure in chemistry and physics. Niels Bohr's model introduces the concept of electrons orbiting the nucleus in specific energy levels, offering a simpler visualization of atoms. Here, we delve into five essential tips that will help you master the Bohr model worksheet answers, enhancing your understanding of atomic theory and improving your performance in science assessments.
1. Comprehend Electron Configurations


The first step to excel in Bohr model worksheet answers is to thoroughly understand electron configurations:
- Understand the Electron Shells: Electrons are arranged in shells or energy levels around the nucleus. The first shell can hold up to 2 electrons, the second up to 8, and this pattern continues with each subsequent shell.
- Know the Order of Filling: Electrons fill the shells from the lowest energy level (closest to the nucleus) upwards. For example, a lithium atom (atomic number 3) has two electrons in the first shell and one in the second shell.
2. Visualize Atomic Structure with Diagrams
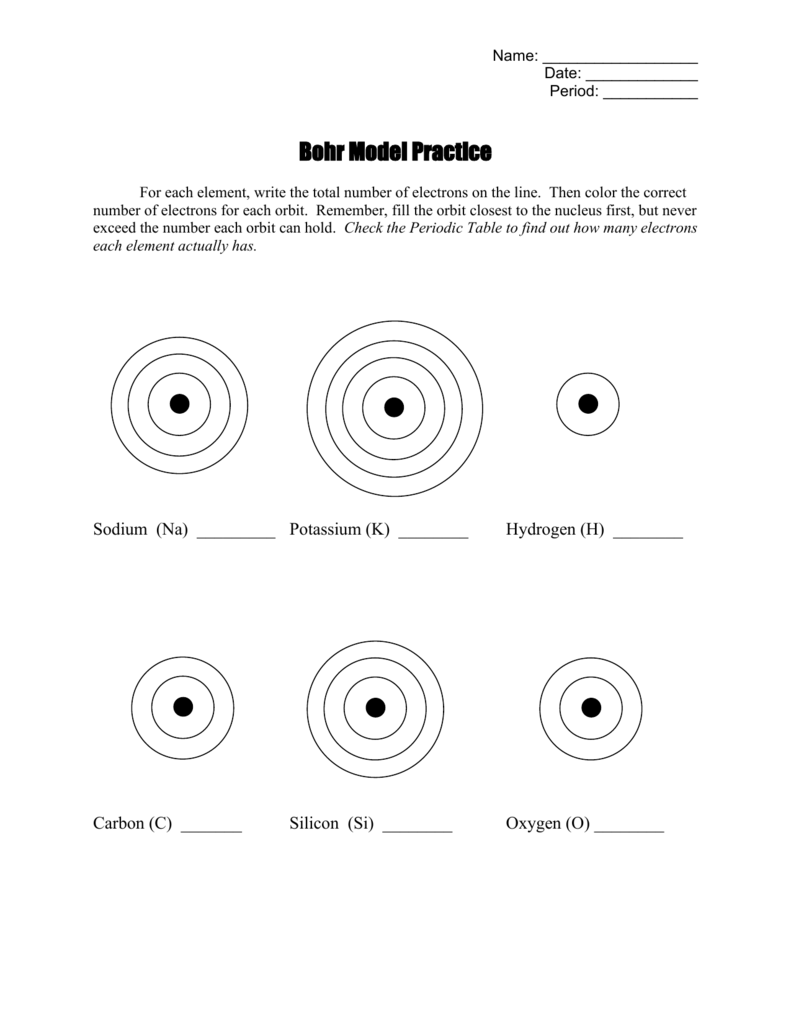
Mastery of drawing Bohr diagrams is crucial:
- Nucleus Placement: Start by placing the nucleus at the center, where protons and neutrons reside.
- Orbital Rings: Draw concentric circles to represent electron shells. The distance between each circle represents an increase in energy level.
- Electron Representation: Place electrons as small dots or ‘e’ symbols around the nucleus in the appropriate energy levels.
⚡ Note: When drawing diagrams, ensure you label each energy level (shell) for clarity, especially in more complex atoms.
3. Use the Periodic Table as Your Cheat Sheet
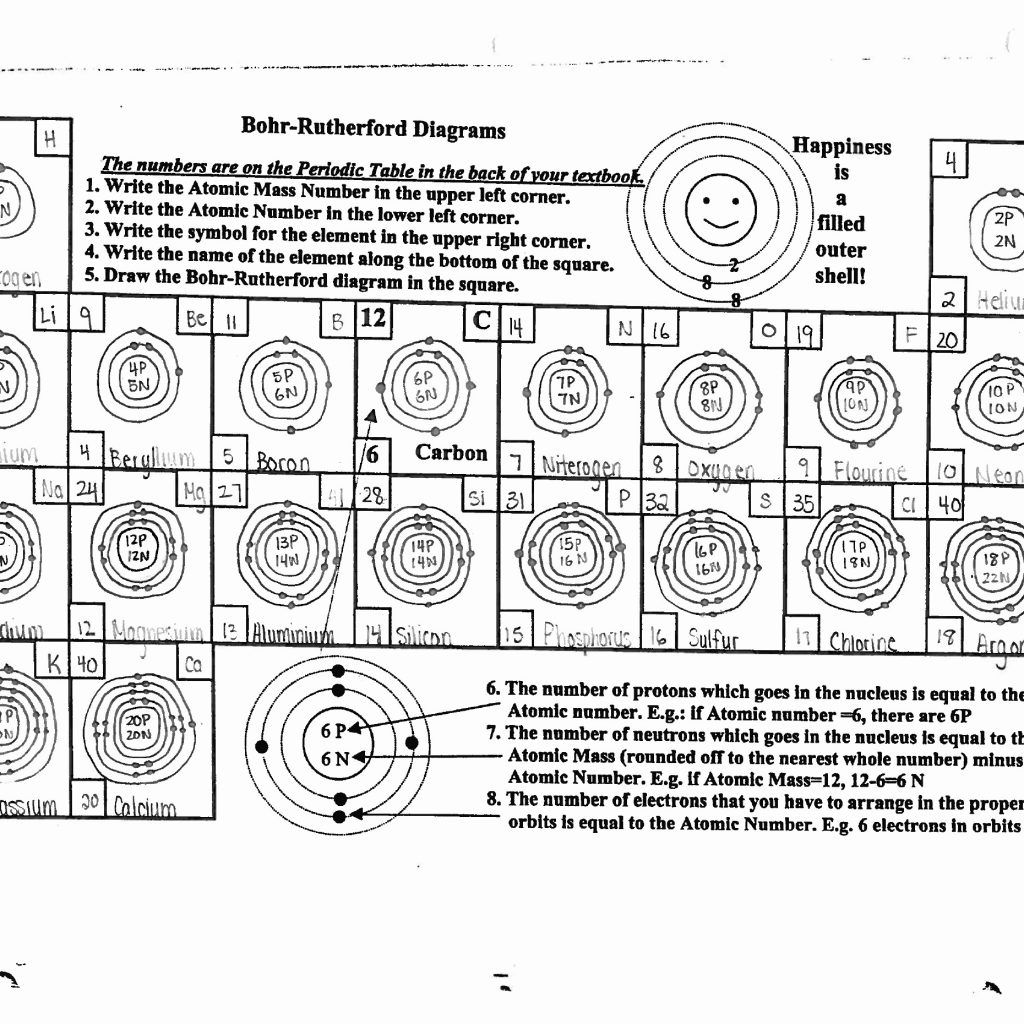
The periodic table is a treasure trove of information for understanding atomic models:
- Atomic Number: This tells you the number of protons, which equals the number of electrons in a neutral atom.
- Element Group: Elements in the same group have similar properties, which can indicate how their electrons are likely arranged.
- Periods: Each period corresponds to the principal quantum number (n), which directly relates to the energy level.
| Element | Atomic Number | Symbol | Electron Configuration |
|---|---|---|---|
| Hydrogen | 1 | H | 1s¹ |
| Helium | 2 | He | 1s² |
| Lithium | 3 | Li | 1s² 2s¹ |

4. Recognize Anomalies
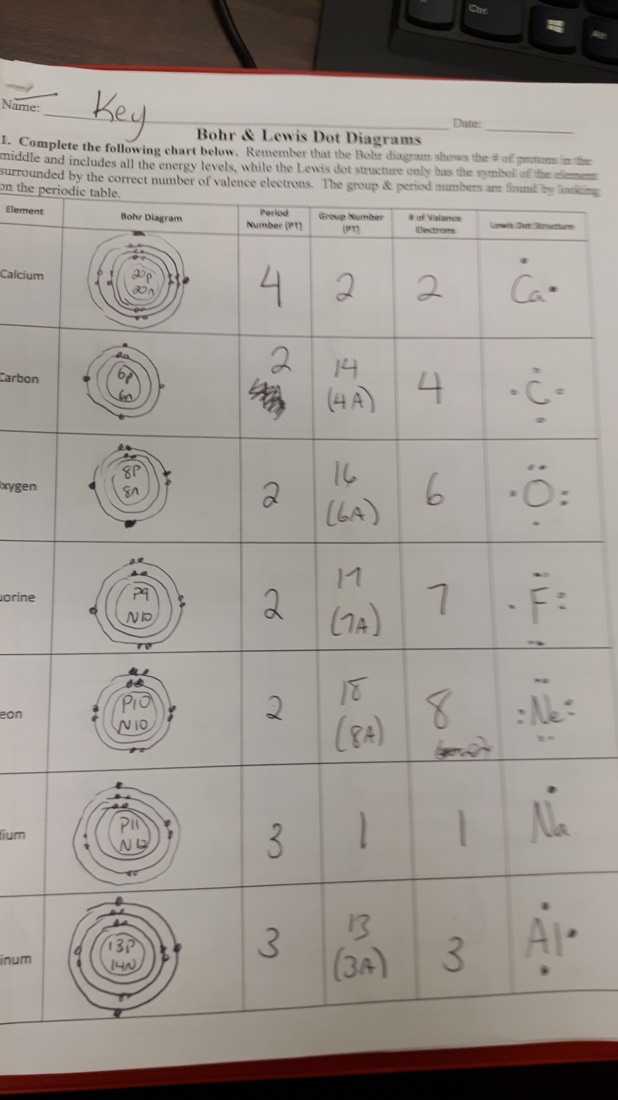
Not all atoms behave strictly according to the simplest rules of the Bohr model:
- Transition Elements: These elements often defy the electron filling order due to electron-electron repulsions.
- Hund’s Rule: Electrons occupy orbitals singly before they pair up, leading to exceptions in electron placement.
- Electronegativity: Atoms with high electronegativity (like fluorine) might slightly alter their electron configurations to achieve stability.
🧐 Note: Transition metals and heavier elements are where you might encounter the most significant deviations from the expected Bohr model predictions.
5. Practice with Bohr Model Worksheets

The key to mastering any topic in science is practice. Here are some ways to use Bohr model worksheets effectively:
- Detailed Diagrams: Draw each atom with meticulous care. Include the nucleus with proton and neutron count, and show electrons in their orbits.
- Variety of Elements: Practice with both light and heavy elements to understand different electron shell capacities and behaviors.
- Worksheet Correction: Check your answers against references or have your instructor review them to ensure accuracy.
In summing up, the Bohr model, while simplified, provides a foundational understanding of atomic structure that remains pivotal in chemistry and physics education. Through comprehending electron configurations, mastering the art of drawing diagrams, utilizing the periodic table, recognizing anomalies, and consistent practice with worksheets, you can enhance your grasp on atomic theory. This knowledge not only aids in academic success but also lays the groundwork for advanced studies in these sciences, enabling you to decode the intricacies of the atomic world with greater ease and precision.
Why is understanding the Bohr model important?

+
Understanding the Bohr model is crucial because it introduces students to the basic principles of atomic structure, laying a foundation for further studies in chemistry and physics. It helps in visualizing how electrons orbit the nucleus and provides a simple model for atomic behavior, which is essential for predicting chemical reactivity and properties of elements.
How do you know how many electrons go into each energy level?
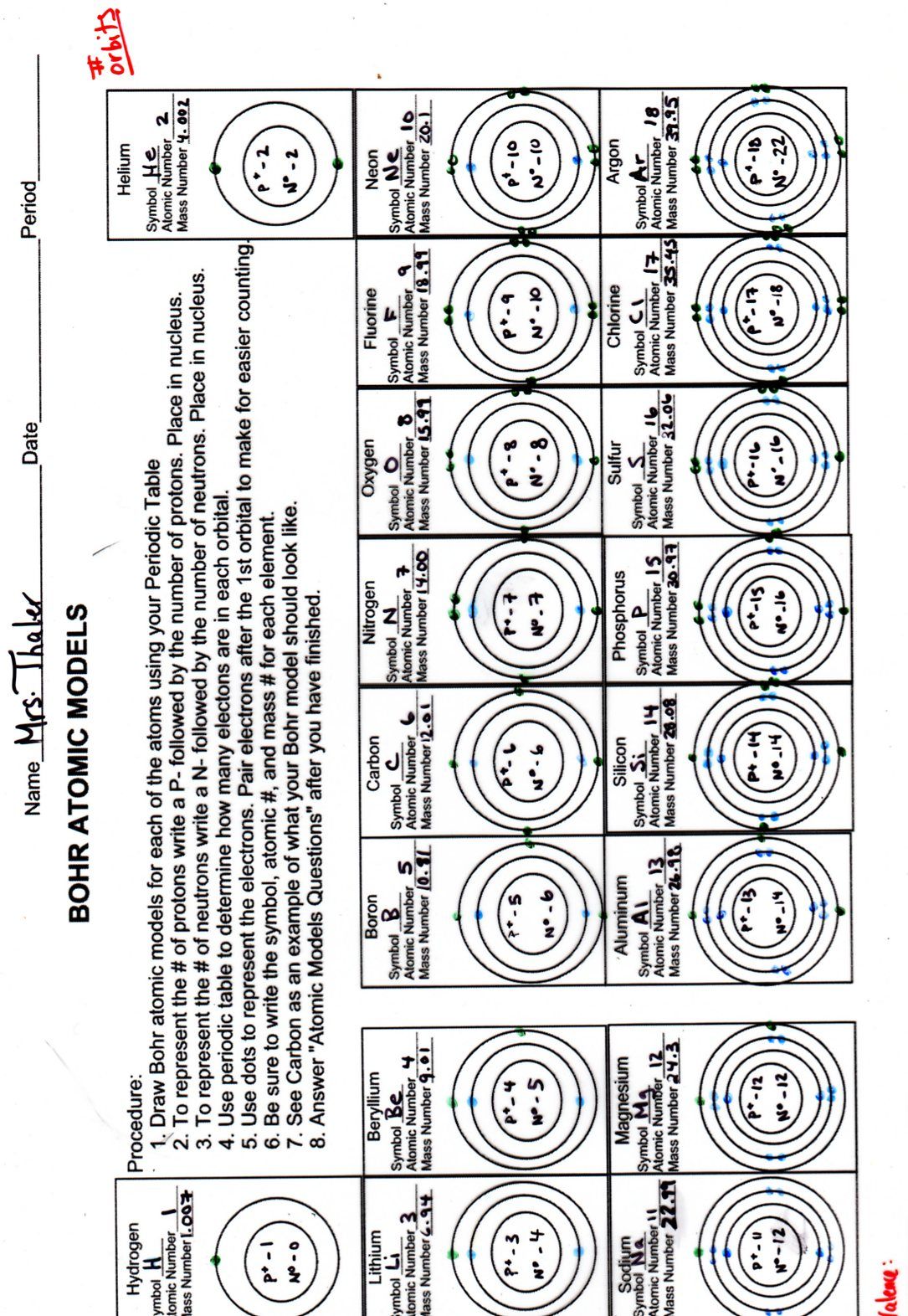
+
The number of electrons in each energy level (n) can be calculated using the formula 2n². For example, the first shell (n=1) can hold up to 2(1)² = 2 electrons; the second shell (n=2) can hold up to 2(2)² = 8 electrons, and so on. However, remember that this is a simplified rule; exceptions exist, especially with heavier elements.
Can you draw Bohr models for ions?

+
Yes, Bohr models can also be drawn for ions. For cations (positively charged ions), you will remove electrons from the outermost shell to achieve a stable configuration, while for anions (negatively charged ions), you add electrons to the outermost shell. Ensure the total number of electrons reflects the ion’s charge, and remember that the number of protons remains the same.