Bohr Model Worksheet Answers: Master Chemistry Easily
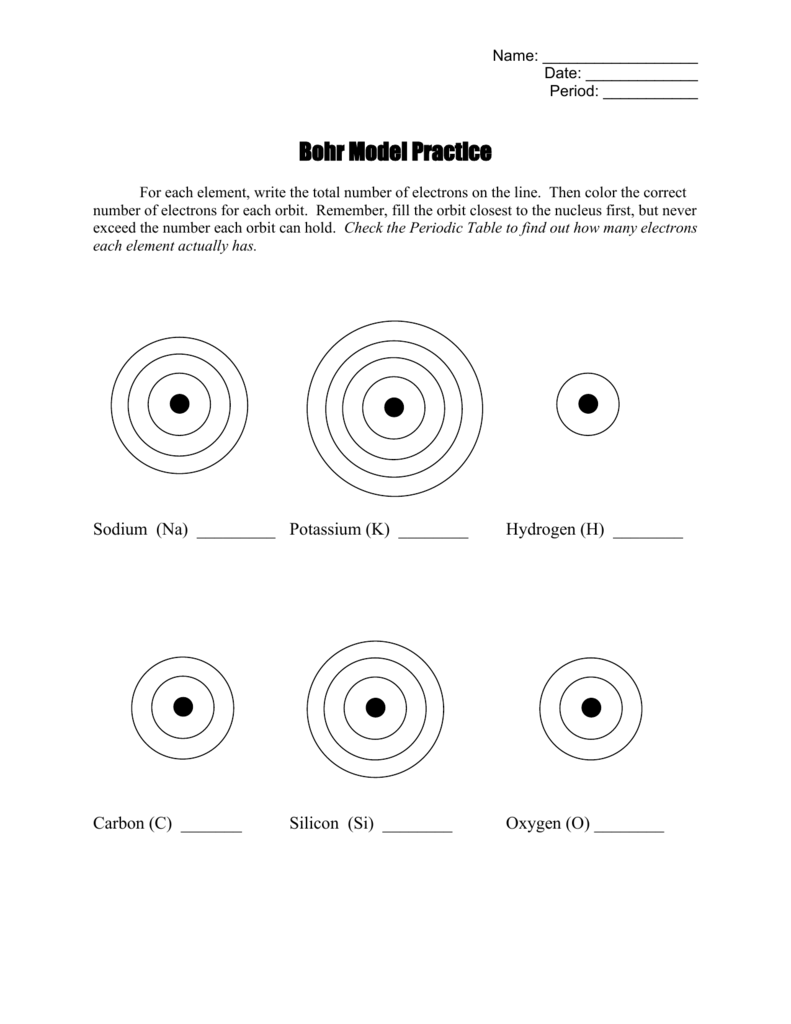
If you are delving into the fascinating world of atomic theory and find the Bohr model of the atom a bit perplexing, you're not alone. Developed by Niels Bohr in 1913, the Bohr model offers a simplified picture of the atom, giving us insight into electron behavior. This guide will walk you through the critical elements of Bohr model worksheets, helping you master chemistry effortlessly.
Understanding the Bohr Model

Before we dive into the worksheets, let's grasp the basics of the Bohr model:
- Orbitals: Electrons revolve around the nucleus in fixed orbits or energy levels.
- Energy Levels: These orbits correspond to different energy levels; electrons absorb or emit energy as they move between these levels.
- Electron Configuration: Each orbit or shell can hold a specific number of electrons, which can be calculated by the formula 2n², where n is the shell number.

Worksheet Breakdown
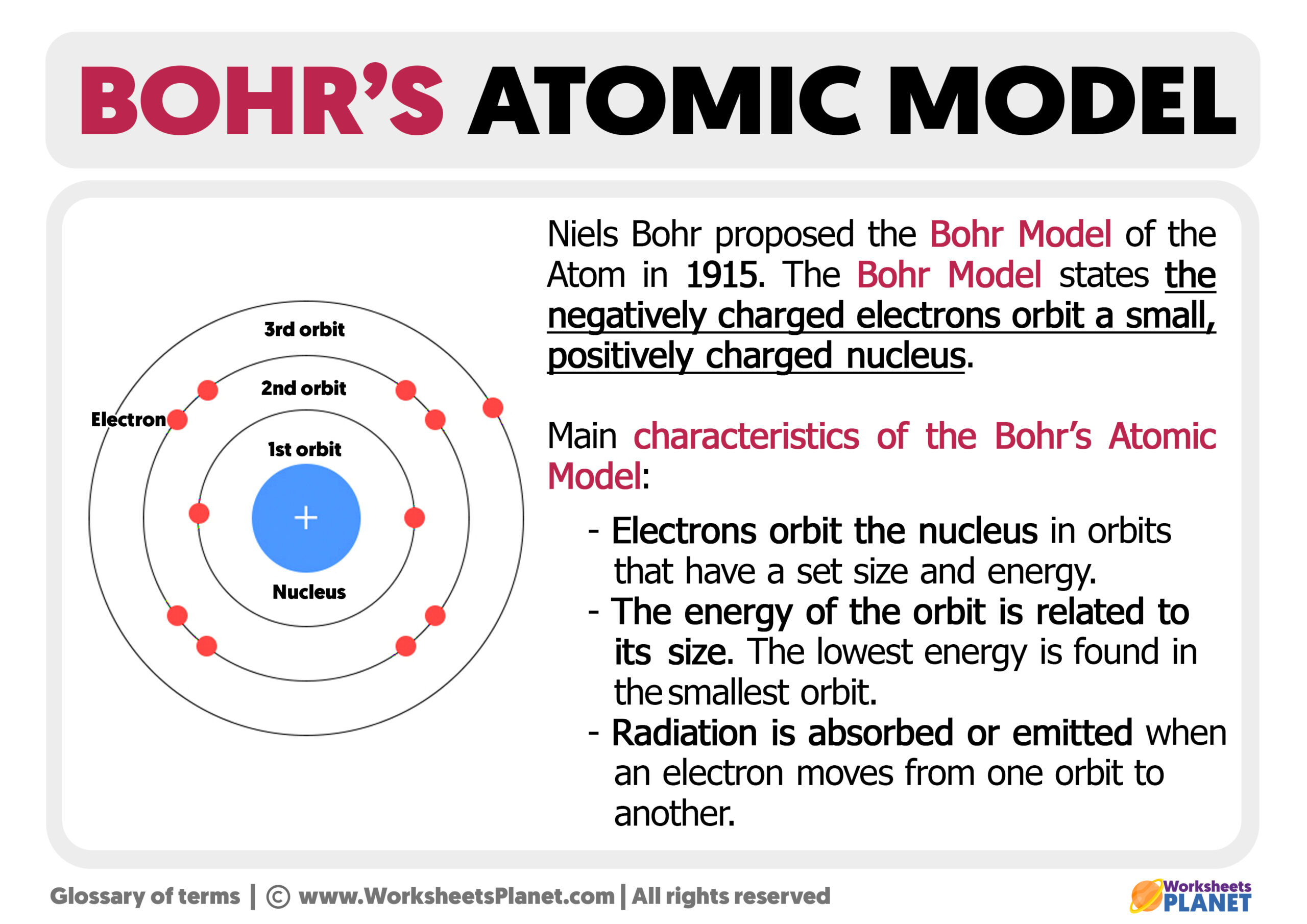
Let's now explore common elements of Bohr model worksheets:
Identifying Elements
Your worksheet might ask you to determine an element based on its atomic number or electron configuration.
- Atomic Number: The number of protons in an atom's nucleus, which equals the number of electrons in a neutral atom.
- Electron Configuration: Writing down how electrons are distributed in various shells. For example, Helium has an electron configuration of 1s².
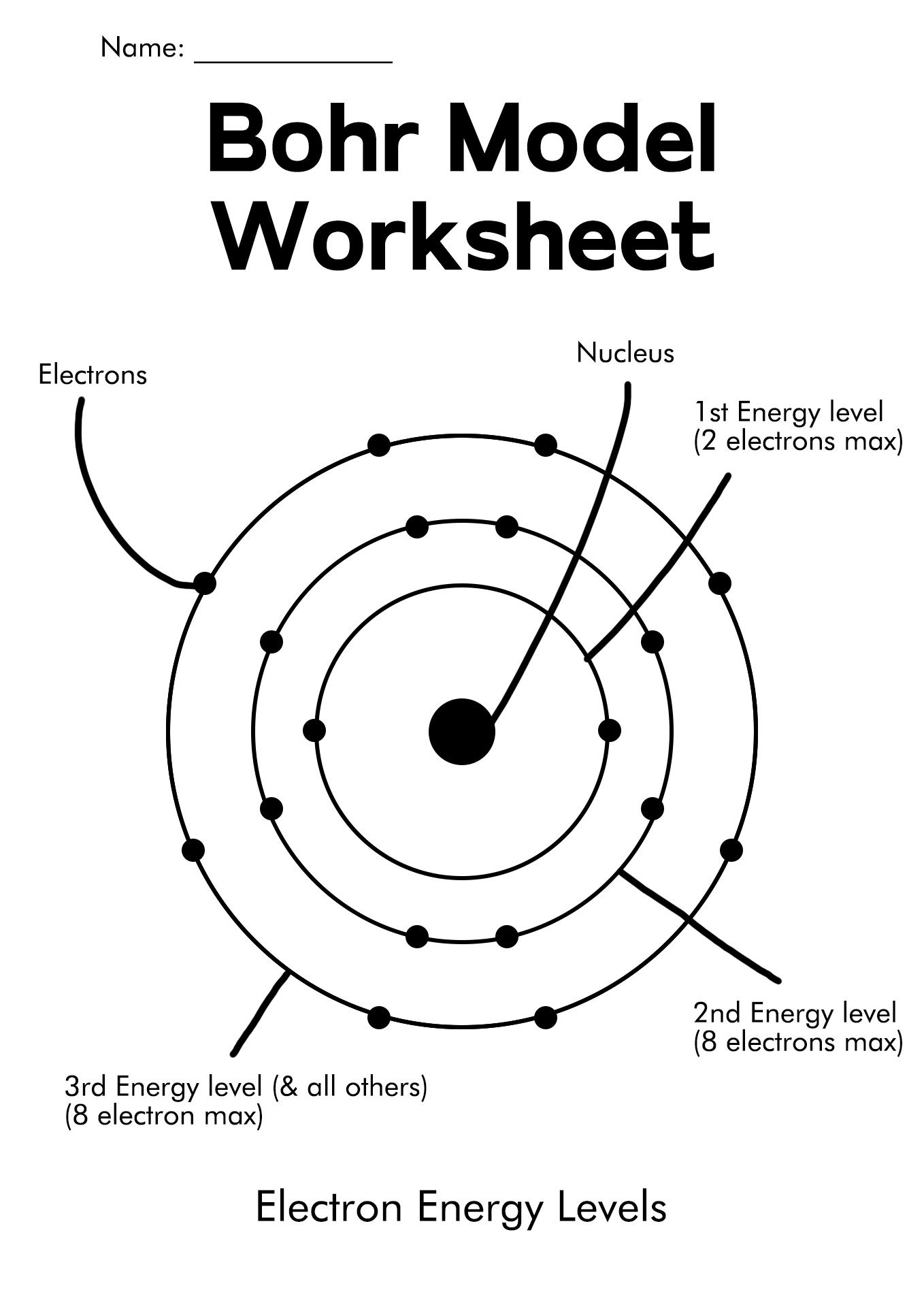
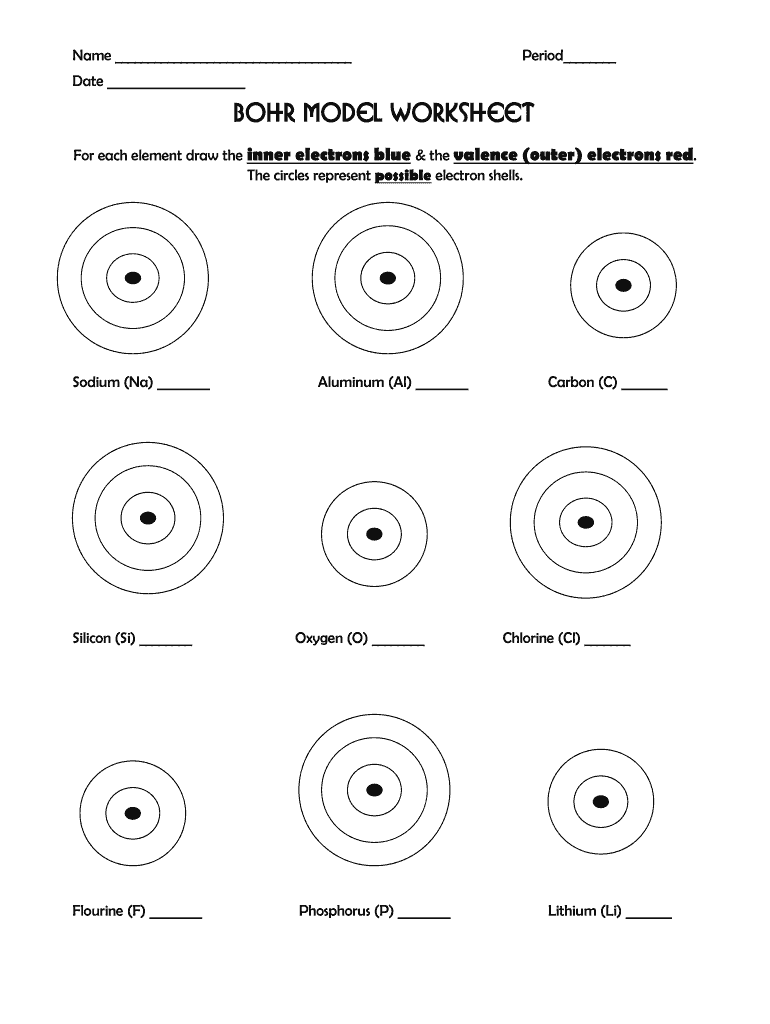
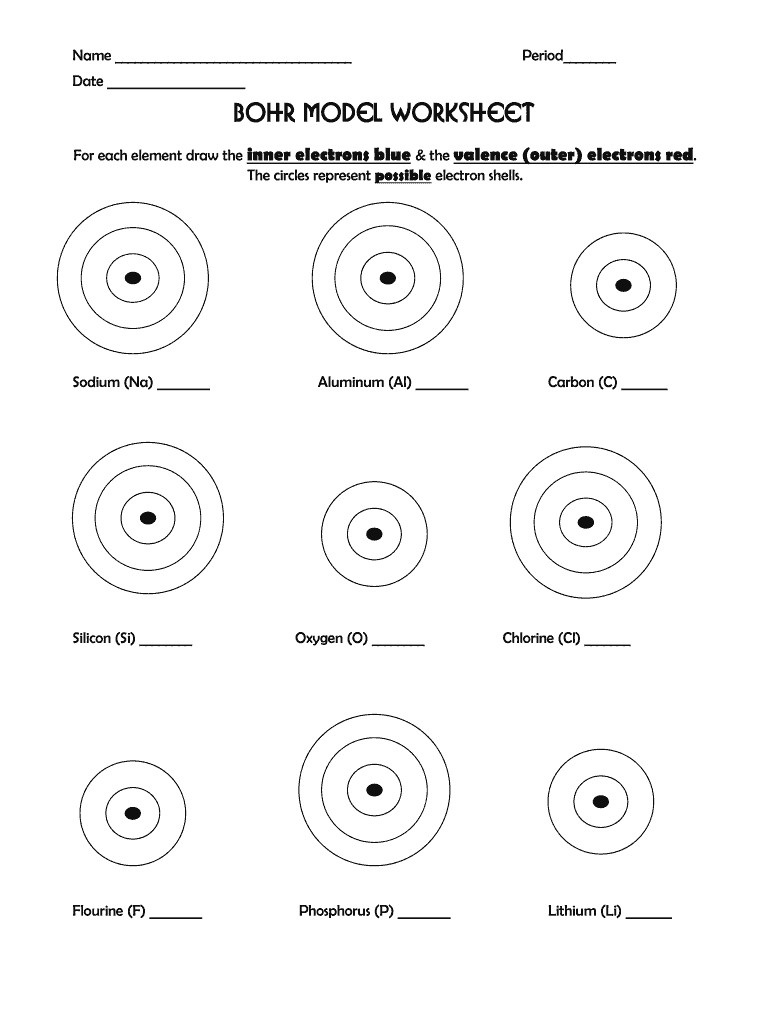
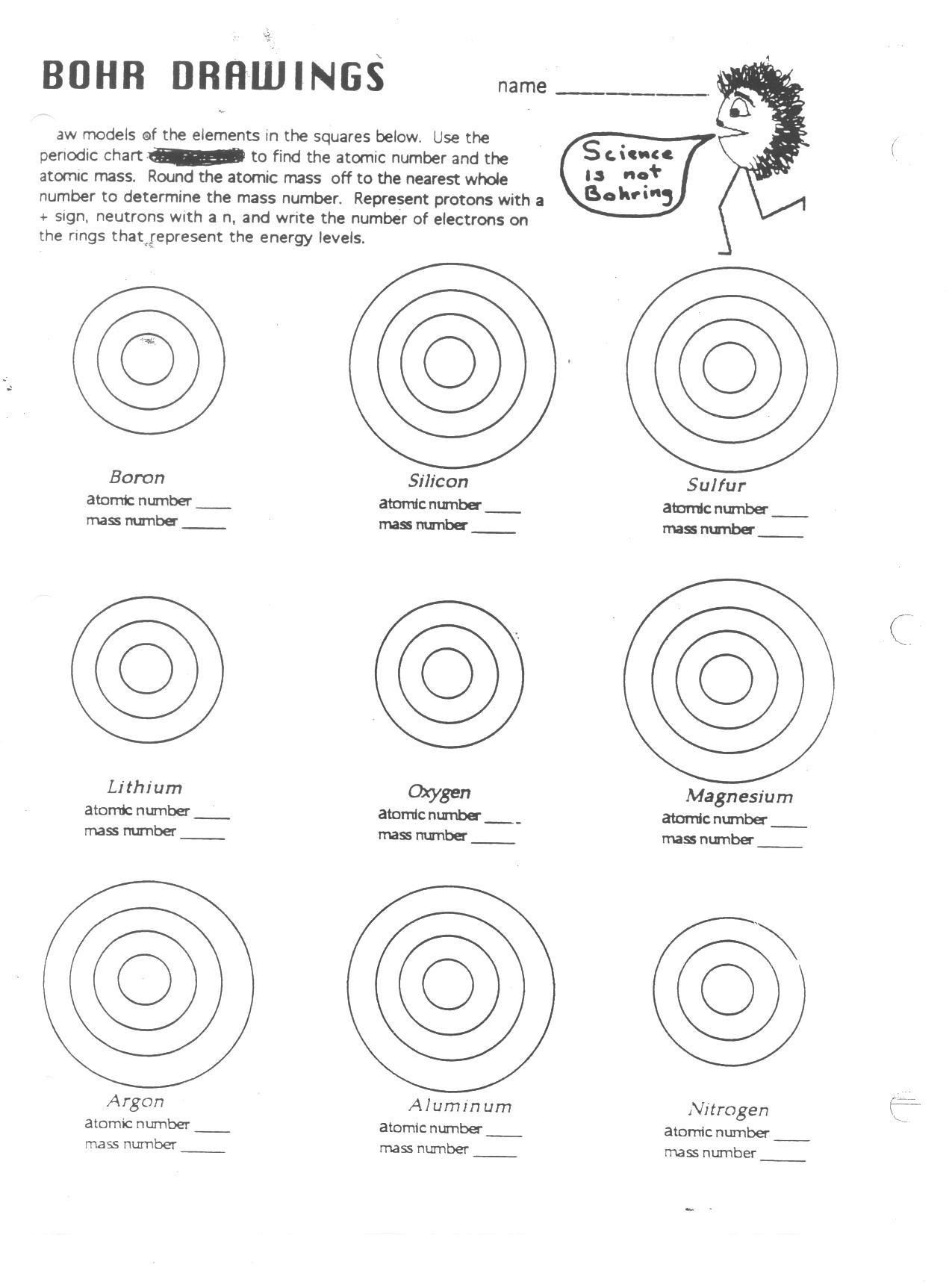
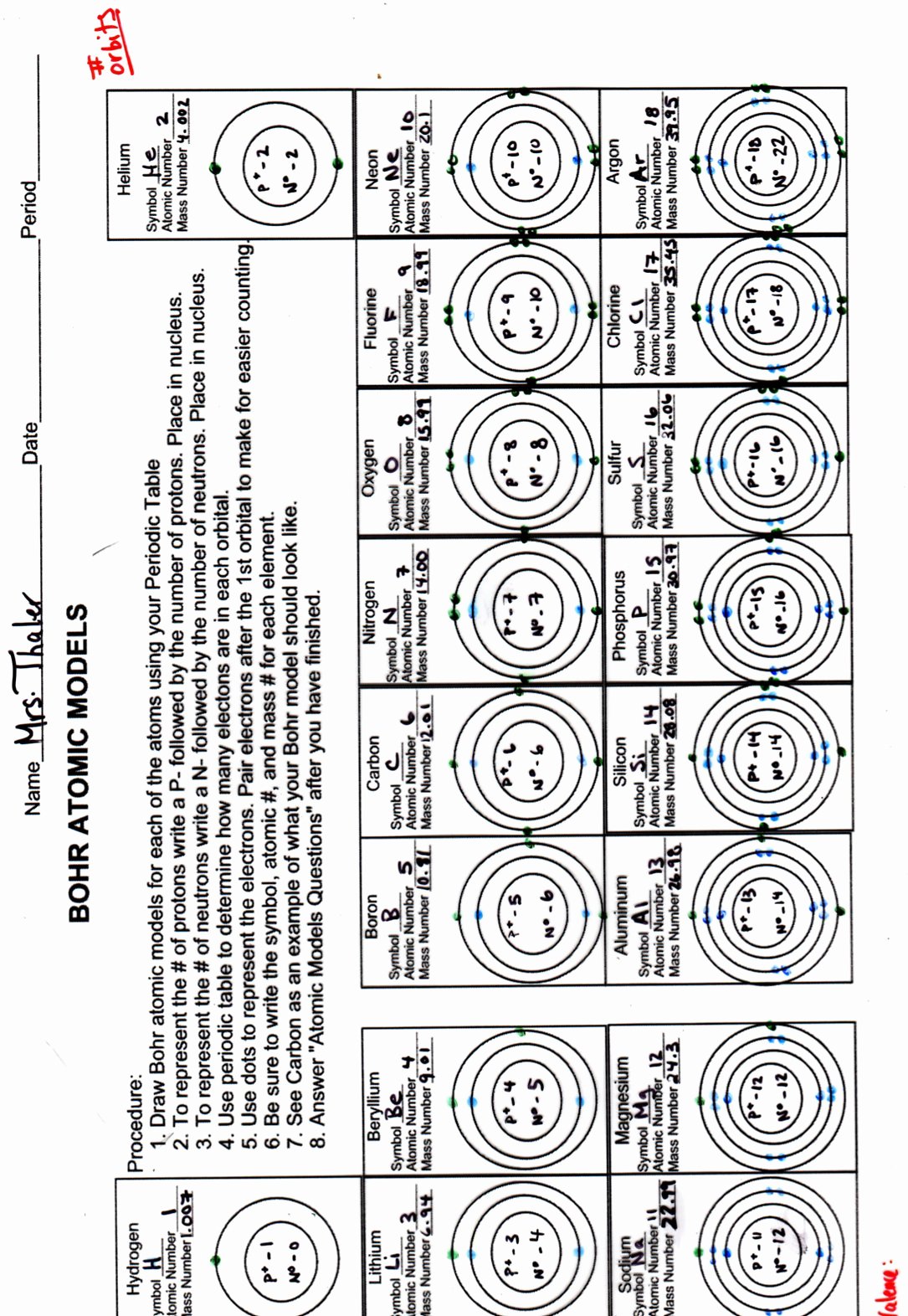
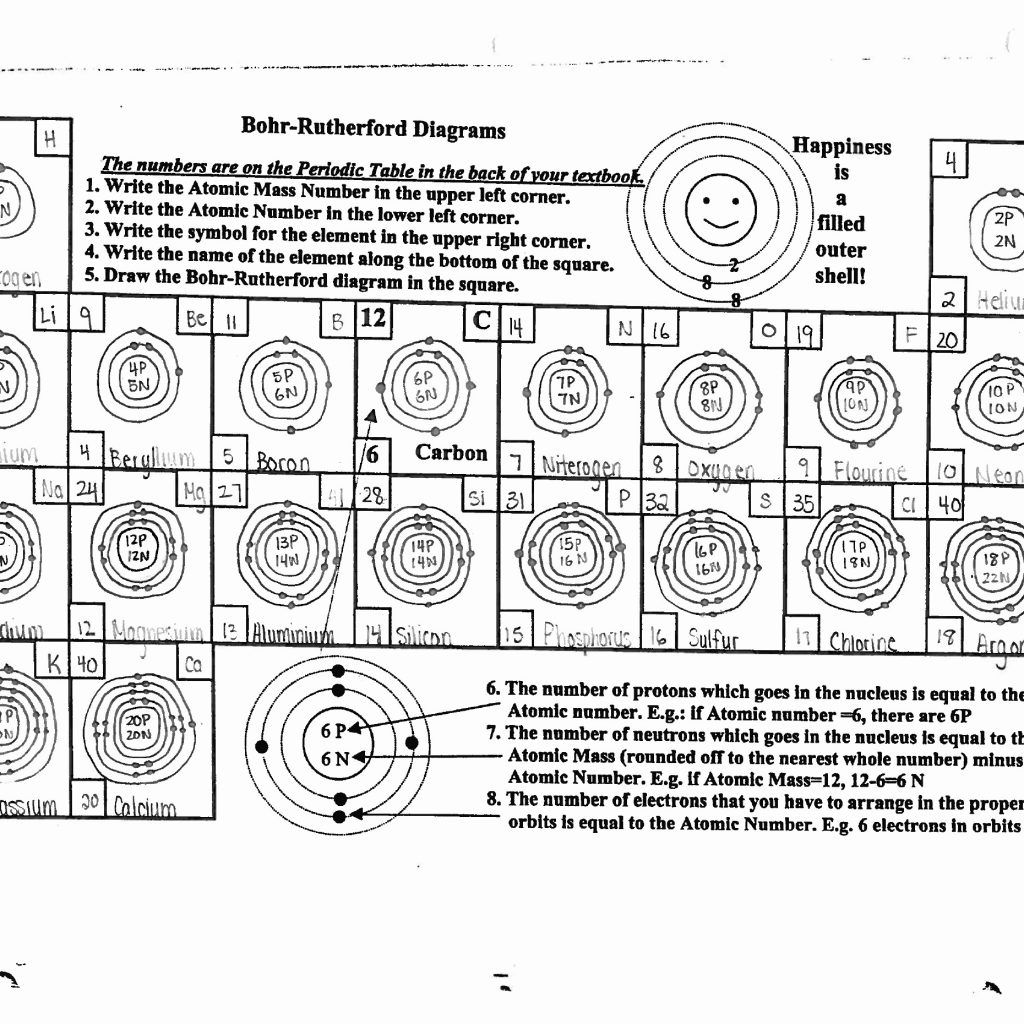
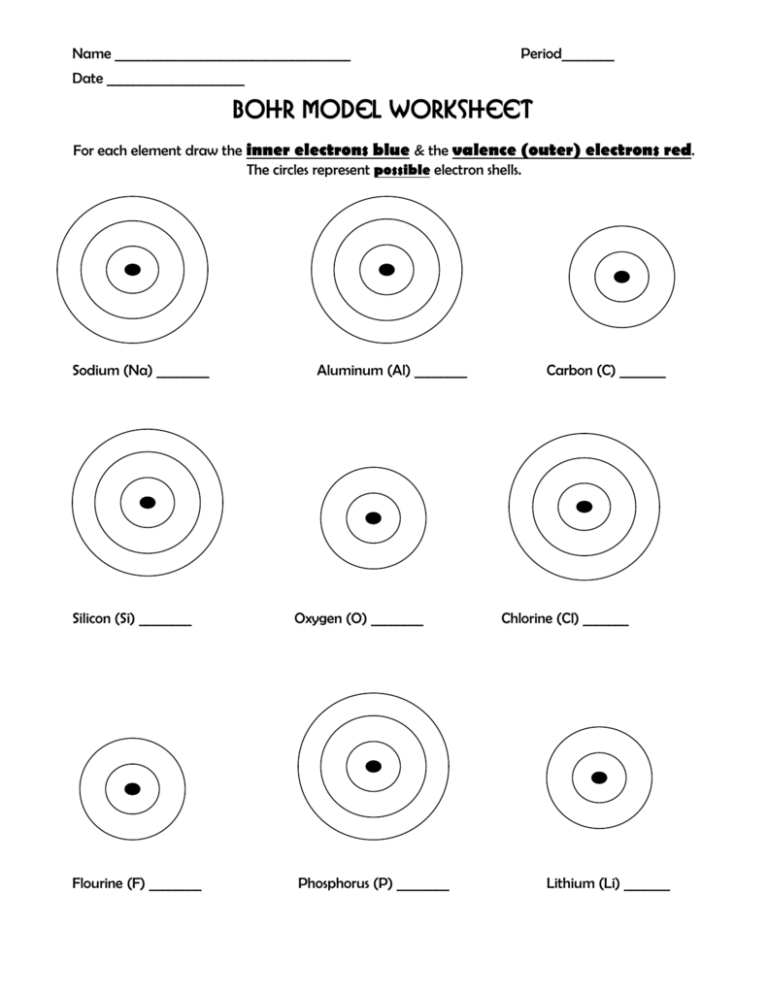
Constructing Bohr Diagrams
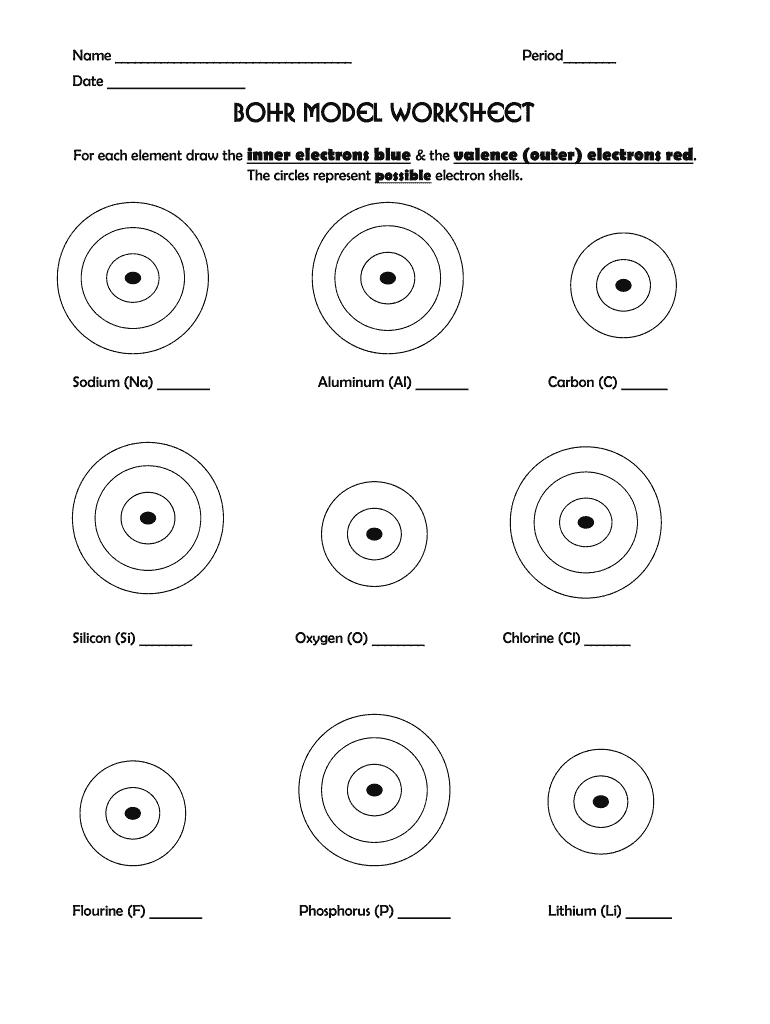
Here's how to construct a Bohr diagram:
- Draw a small circle to represent the nucleus; label it with the element's symbol and atomic number.
- Add rings around the nucleus to represent electron shells. The first shell (n=1) holds 2 electrons, the second (n=2) can hold up to 8, and so forth.
- Place the electrons (dots or x’s) in each shell, adhering to the 2n² rule.
Calculating Electron Energies
Some questions might involve calculating the energy difference when electrons move between orbits:
- Use the formula: ΔE = -R_H (1/n_f² - 1/n_i²), where R_H is the Rydberg constant (2.18 × 10^-18 J).
- n_f represents the final energy level, while n_i is the initial energy level.
💡 Note: While the Bohr model simplifies atomic structure, it’s worth noting that electrons don't occupy fixed orbits in reality but rather exist in probability clouds called orbitals.
Key Features and Limitations
The Bohr model has significant benefits:
- It provides a visual representation of electron distribution.
- It explains the emission and absorption of energy by atoms (spectral lines).
However, there are limitations:
- The model fails to account for electron-electron interactions.
- It doesn't apply to heavier elements effectively.
- Modern quantum mechanics has introduced more complex and accurate models like quantum numbers and subshells.
Despite these limitations, mastering the Bohr model can give you a foundational understanding of atomic structure, invaluable for further studies in chemistry.
Mastering Bohr Model Worksheets
To excel in Bohr model worksheets, follow these tips:
- Practice: The more you work with Bohr diagrams, the more intuitive they become.
- Learn from Mistakes: Analyze incorrect answers to understand why you went wrong.
- Relate: Connect the concepts to real-world applications, like atomic spectra.
- Visualize: Visual aids and molecular kits can make learning three-dimensional and tangible.
With these insights, you can master the Bohr model of the atom, making chemistry an exciting and understandable subject.
Ultimately, the Bohr model worksheet serves not only as a way to learn about atomic structure but also as a tool to ignite curiosity and deepen your understanding of chemistry. By focusing on the strengths and limitations of the Bohr model, you can build a comprehensive knowledge base that will serve you well in your academic and scientific journey.
What is the main purpose of the Bohr model?
+
The Bohr model’s primary purpose is to give a simplified representation of how electrons are arranged around an atom’s nucleus, showing the quantized energy levels of electrons.
Why does the Bohr model work for hydrogen but not for heavier elements?
+
The simplicity of hydrogen’s electron distribution makes the Bohr model effective. However, as atomic number increases, electron-electron interactions become more complex, leading to electron spin, angular momentum, and electron pair repulsion, which the model doesn’t account for.
Can you use the Bohr model to predict chemical behavior?
+
To a limited extent. It explains electron movement and energy absorption/emission, but for a thorough understanding of chemical reactions and bonding, one must resort to quantum mechanics and molecular orbitals.
What are the common mistakes in drawing Bohr model diagrams?
+
Errors typically include filling shells with too many or too few electrons, neglecting the 2n² rule, and sometimes skipping shell numbers.