5 Easy Steps to Master Bohr Model Drawing
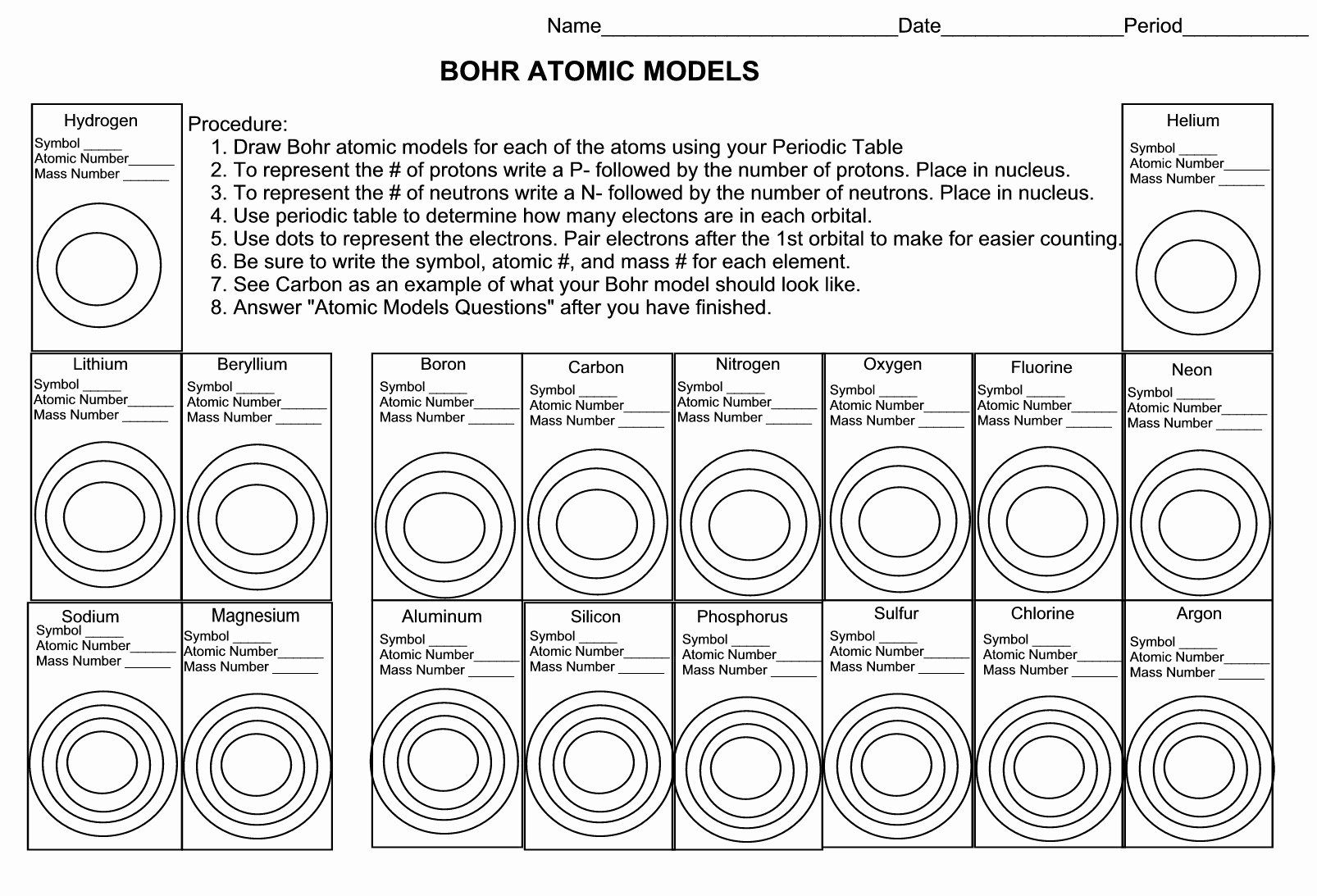
Understanding the atomic structure has been fundamental to the progress in chemistry and physics. One of the earliest and most influential models was the Bohr Model, introduced by Niels Bohr in 1913. It revolutionized our understanding of atoms by providing a visual representation that simplifies complex quantum mechanics into something more tangible. This blog post will guide you through the process of mastering Bohr Model drawing with five easy steps, ensuring you grasp not just how to draw them, but also comprehend their significance in the scientific community.
1. Understand the Basics of the Bohr Model


Before you can master drawing Bohr Models, it’s essential to understand what they represent. Here’s a quick overview:
- The Bohr Model depicts electrons orbiting the nucleus in fixed circular orbits.
- The nucleus, which is at the center, contains protons and neutrons.
- Each orbit corresponds to a specific energy level for electrons, denoted as K, L, M, N, etc.
To effectively use the Bohr Model:
- Use concentric circles to represent energy levels, each labeled.
- The number of electrons in each shell can be calculated using the 2n² rule, where n is the shell number.
- Place the nucleus at the center with a small dot for each proton and neutron.
📘 Note: The Bohr Model is a simplified representation. While useful for visualization, it doesn’t show the probability clouds or orbitals of quantum mechanics.
2. Gather Your Materials


Mastering Bohr Model drawing requires some basic supplies:
- Paper
- Pencil or pen
- Colored pencils or markers (for representing different energy levels)
- A ruler
- A compass (optional but helpful for perfect circles)
Having the right tools will make your Bohr Model both visually appealing and more accurate.
3. Determine the Number of Electrons, Protons, and Neutrons
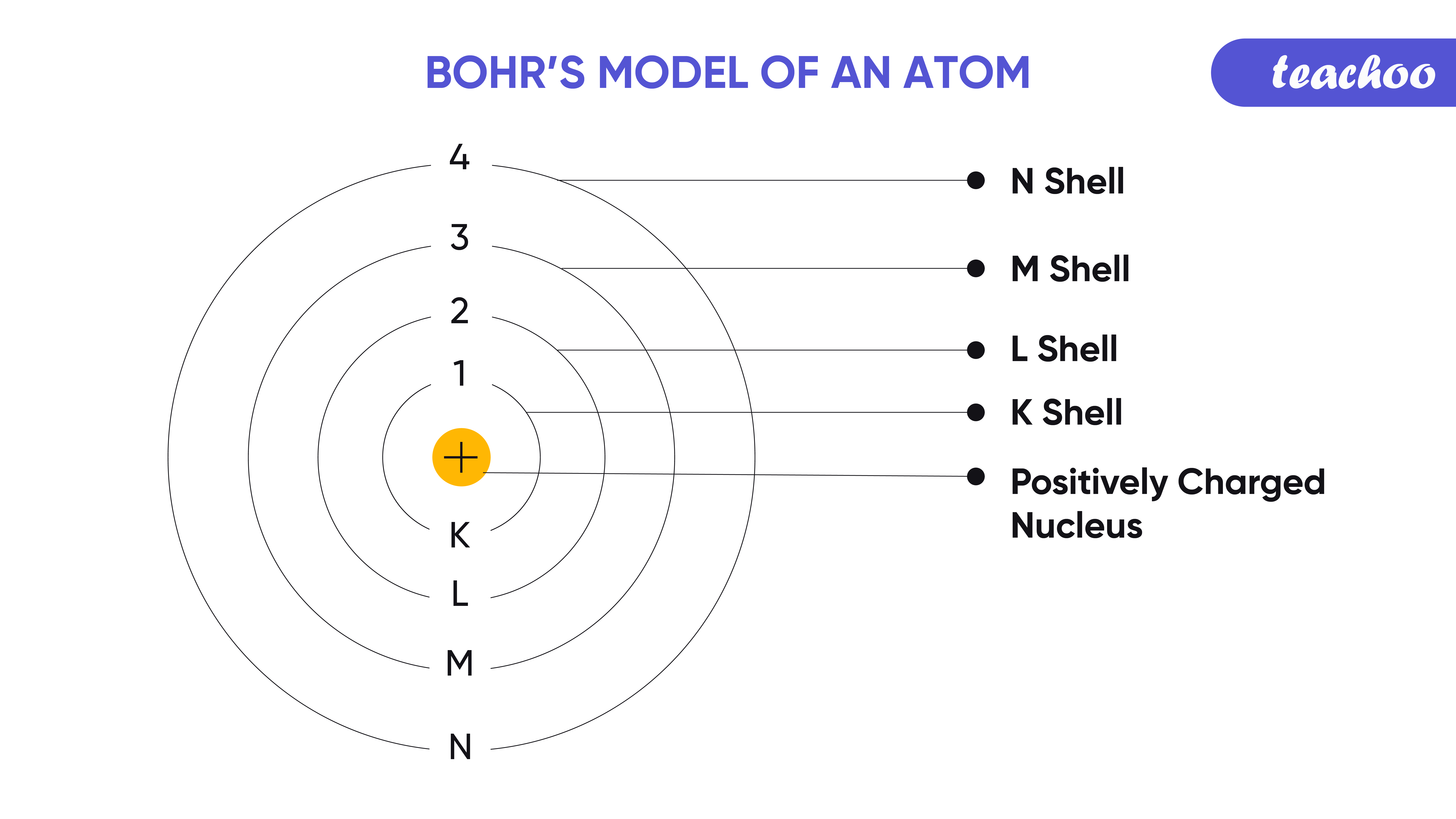
To draw a Bohr Model, you’ll first need to:
- Identify the atomic number of the element, which is the number of protons (also indicating the number of electrons).
- Determine the mass number to find the total number of protons and neutrons. The difference between these is the number of neutrons.
Here’s how you can calculate electrons in each shell:
| Energy Level | Number of Electrons |
|---|---|
| 1 (K) | 2 |
| 2 (L) | 8 |
| 3 (M) | 18 |
| 4 (N) | 32 |

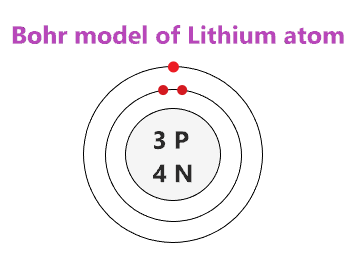
4. Construct Your Bohr Model


Now, let’s construct the Bohr Model:
- Start with the nucleus. Draw a circle at the center of your paper to represent the nucleus. Inside, draw small dots for each proton (positively charged) and neutron (neutral).
- Draw concentric circles for each energy level. Start with the first energy level (K shell) closest to the nucleus. Each subsequent circle should be larger.
- Add electrons (small dots) around these circles, remembering:
- The first shell can hold a maximum of 2 electrons.
- The second shell can hold up to 8 electrons, and this follows the trend of the periodic table.
- Ensure electrons are distributed according to the Aufbau principle or you can use the 2n² rule mentioned earlier for a quick calculation.
📘 Note: Remember that you’ll start filling shells from the inside out, and electrons will fill the lowest energy level first before moving to the next one.
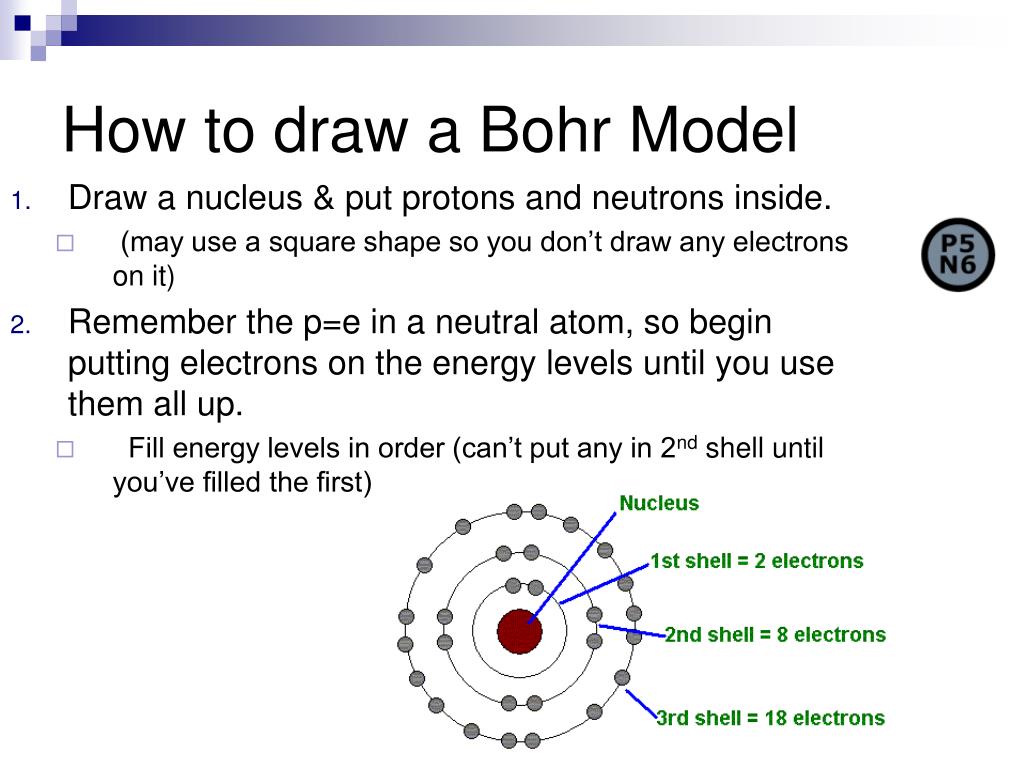
5. Refine and Enhance Your Model
To make your Bohr Model not just accurate but also engaging:
- Color-code your circles to represent different energy levels. A common convention is to use different colors to show progression outward from the nucleus.
- Use arrows or lines to indicate electron spin or the pairing of electrons.
- Label each shell with its energy level (K, L, M, etc.) and the number of electrons present.
- If possible, include the atomic symbol, atomic number, and atomic mass below the Bohr Model.
In summary, mastering the art of drawing Bohr Models involves understanding the basic concepts, having the right materials, calculating the number of subatomic particles, constructing the model accurately, and then refining it for visual and educational impact. With these steps, you can confidently represent and comprehend atomic structure, appreciate its historical significance in science, and even apply these concepts to further your studies in chemistry or physics.
Why is the Bohr Model still used if it’s not perfectly accurate?

+
The Bohr Model, while simplistic, provides a visually intuitive way to introduce the concept of atomic structure to students. It also highlights the quantized nature of energy levels, which is fundamental in quantum physics.
Can the Bohr Model represent all elements?
+
Yes, but it becomes less representative for heavier elements due to the complex electron configurations and the breakdown of the simple Bohr picture. However, it remains a useful teaching tool.
How can I tell if my Bohr Model is correct?
+
Check the number of protons, neutrons, and electrons you’ve placed on your model. Ensure they match the element’s atomic number, mass number, and electron configuration as per the periodic table and the 2n² rule for electron distribution.



