Bohr Model Diagrams: Easy Worksheet Answers Guide

Understanding the structure of atoms is fundamental in the study of chemistry and physics, and one of the most visually intuitive models for this purpose is the Bohr Model. Developed by Niels Bohr in 1913, this model presents atoms as a nucleus surrounded by orbiting electrons, akin to planets orbiting a sun. This blog post provides a comprehensive guide on how to create Bohr Model diagrams with answers, ensuring students and enthusiasts alike can master this key concept in atomic theory.
What is a Bohr Model Diagram?

A Bohr Model Diagram is a simplified representation of an atom, where:
- The nucleus is depicted as a small, dense core containing protons and neutrons.
- Electrons orbit the nucleus in fixed, defined shells or energy levels.
Each shell can hold a specific number of electrons:
- First Shell (n=1) holds up to 2 electrons.
- Second Shell (n=2) holds up to 8 electrons.
- Third Shell (n=3) holds up to 18 electrons, but for simplicity, we often use 8 for the first three levels in educational settings.
💡 Note: Remember, while the Bohr model is useful for basic understanding, it’s a simplification. Modern atomic theory shows electrons as clouds of probability rather than fixed orbits.
How to Draw Bohr Model Diagrams

Step-by-Step Instructions

- Identify the Atom: Choose the element you wish to diagram. You’ll need to know its atomic number.
- Draw the Nucleus: Sketch a circle in the center of your diagram to represent the nucleus.
- Add Protons and Neutrons: Inside the nucleus, write the number of protons and neutrons (if given). For example, Hydrogen has one proton (and can have no or one neutron).
- Draw Electron Shells: Begin with the innermost shell (n=1) and work outwards. Remember the capacity of each shell:
- First Shell: 2 electrons
- Second Shell: 8 electrons
- Third Shell: Typically 8 for educational purposes
- Place Electrons: Fill each shell with electrons until the number of electrons equals the atomic number of the element. Use dots or small circles to represent electrons. Start filling from the innermost shell, moving outwards.
Example: Oxygen

Let’s illustrate the Bohr Model for an oxygen atom:
- Atomic Number = 8 (8 protons).
- Oxygen’s neutral atom would have 8 electrons.

Here, we’ve:
- Drawn the nucleus with 8 protons.
- Added two electrons in the first shell.
- Placed the remaining 6 electrons in the second shell.
Answers Guide for Common Elements

| Element | Atomic Number | Protons | Electrons | First Shell | Second Shell | Third Shell |
|---|---|---|---|---|---|---|
| Hydrogen (H) | 1 | 1 | 1 | 1 | 0 | 0 |
| Helium (He) | 2 | 2 | 2 | 2 | 0 | 0 |
| Nitrogen (N) | 7 | 7 | 7 | 2 | 5 | 0 |
| Oxygen (O) | 8 | 8 | 8 | 2 | 6 | 0 |
| Neon (Ne) | 10 | 10 | 10 | 2 | 8 | 0 |
| Sodium (Na) | 11 | 11 | 11 | 2 | 8 | 1 |
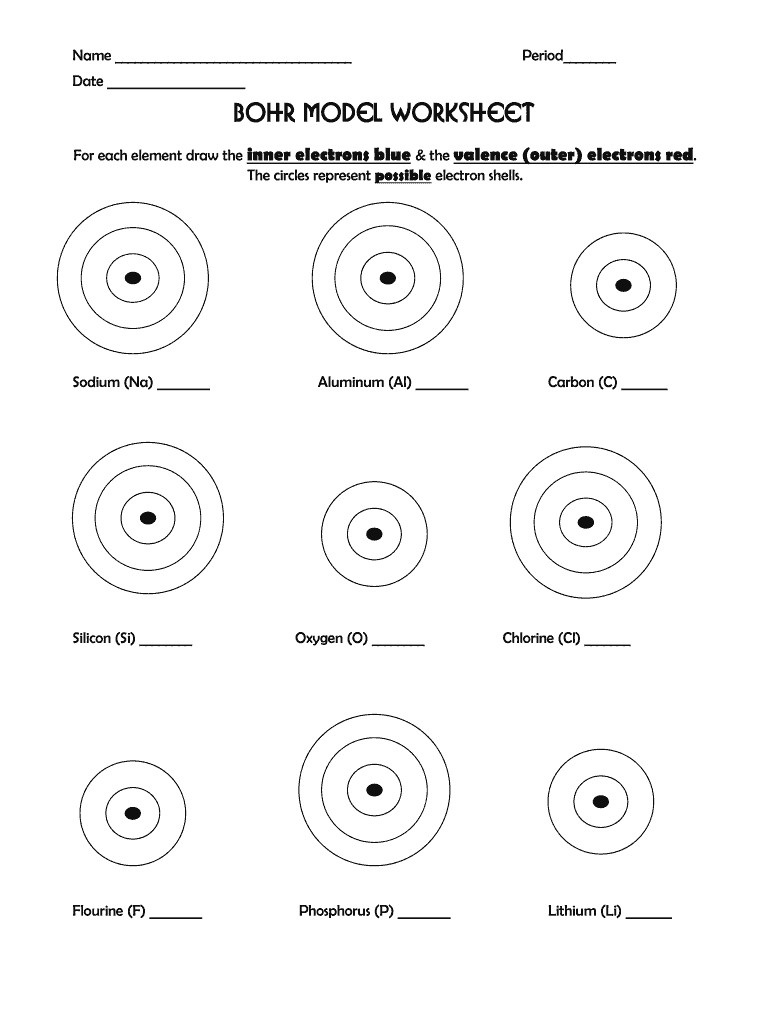
This table should serve as a quick reference for students to understand the distribution of electrons in Bohr models for common elements.
The Bohr Model, although simplified, provides an excellent foundation for understanding atomic structure. By visualizing the electron shells and the arrangement of protons and neutrons, learners can easily grasp how atoms differ from one another, how they interact, and why they form bonds. While modern theories like quantum mechanics have superseded it in terms of accuracy, the Bohr model remains invaluable for educational purposes due to its simplicity and accessibility. By practicing with the guide and example provided, anyone from students to chemistry enthusiasts can confidently construct and understand Bohr diagrams, enhancing their knowledge of atomic theory.
Why is the Bohr Model important despite its simplifications?

+
The Bohr Model is vital in educational settings as it provides a visual and easy-to-understand foundation for learning about atomic structure. It helps in understanding basic principles of atomic bonding, ionization, and electron behavior without the complexity of modern quantum mechanics.
How accurate is the Bohr Model compared to modern atomic theories?

+
The Bohr Model is a simplification that doesn’t account for electron orbitals and their probability clouds as described by quantum mechanics. However, for educational purposes and a basic understanding of atomic structure, it’s quite accurate and useful.
What are the limitations of the Bohr Model?

+
The Bohr Model assumes electrons travel in fixed orbits, which isn’t true. It can’t predict all atomic properties or accommodate multi-electron systems accurately, and it fails to explain spectral lines from heavier elements beyond hydrogen.
Can I use the Bohr Model for all elements?

+
Yes, for educational purposes and simplicity, the Bohr Model can be applied to all elements. However, for precise atomic behavior and properties, especially in more complex atoms, other models or theories might be necessary.
How can I check if my Bohr Model diagram is correct?

+
Check the total number of electrons against the element’s atomic number. Ensure that electrons are filled in shells correctly, following the 2-8-8 rule for the first three levels. Also, verify the position and number of protons and neutrons in the nucleus.