5 Proven Ways to Master Bohr Atomic Models
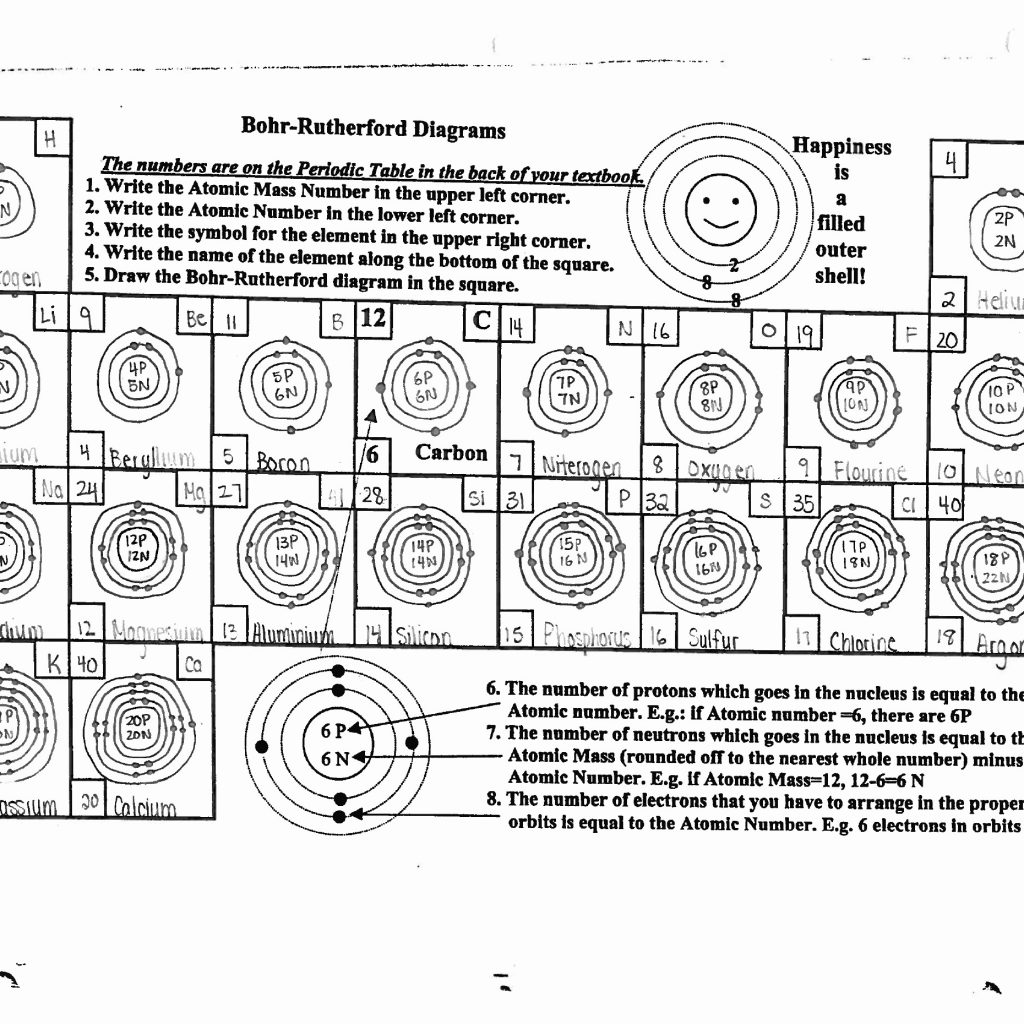
Exploring the world of atomic models, particularly the Bohr model, offers a fascinating journey into the heart of chemistry and physics. Developed by Niels Bohr in 1913, this revolutionary model laid the foundation for our understanding of atomic structure, electron configuration, and the behavior of subatomic particles. In this detailed guide, we'll delve into five proven methods to master Bohr's atomic model, offering insights into its intricacies and applications in modern science.
Understanding the Basics of Bohr’s Model
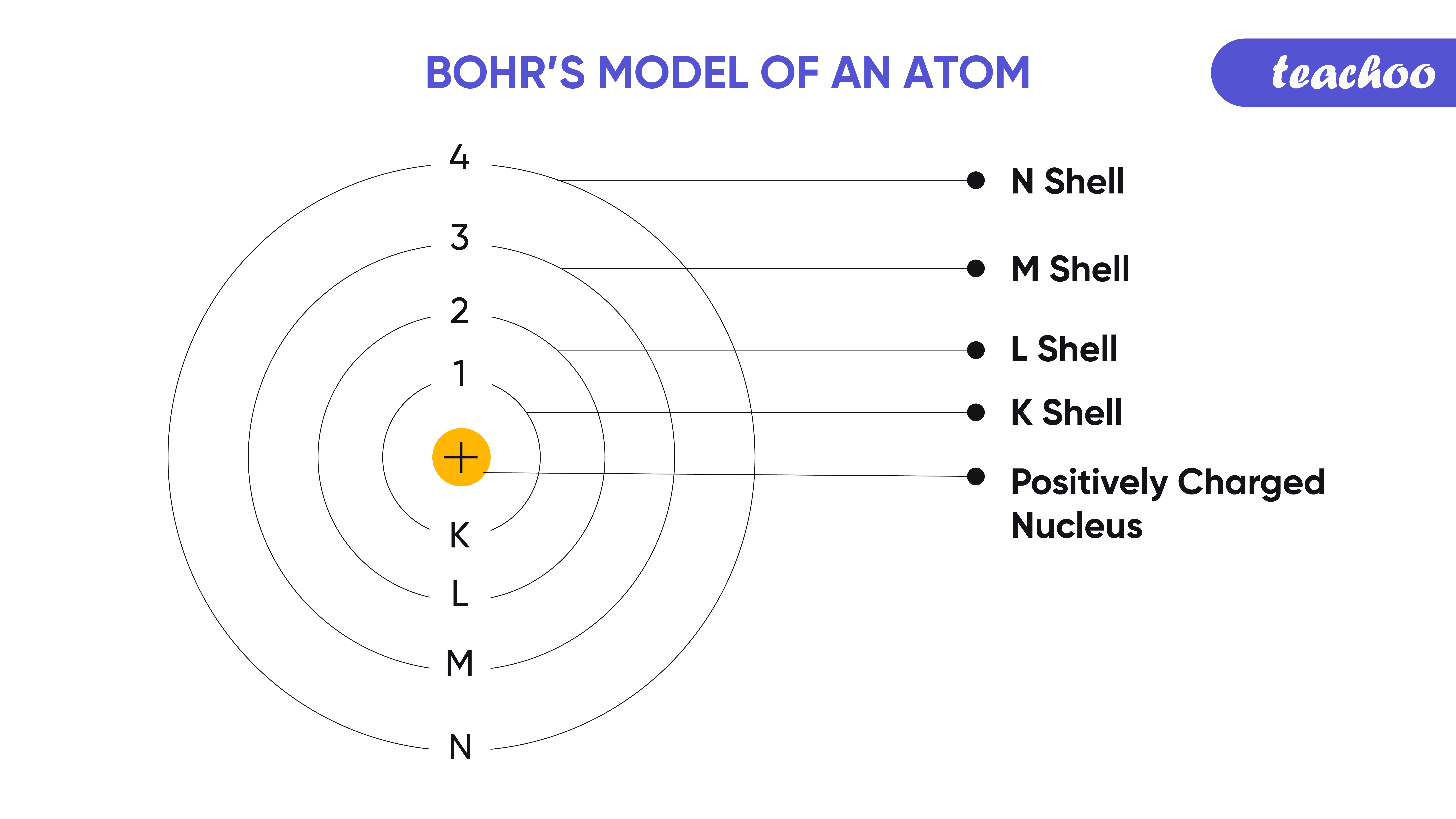
The first step in mastering the Bohr atomic model is to grasp its fundamental principles:
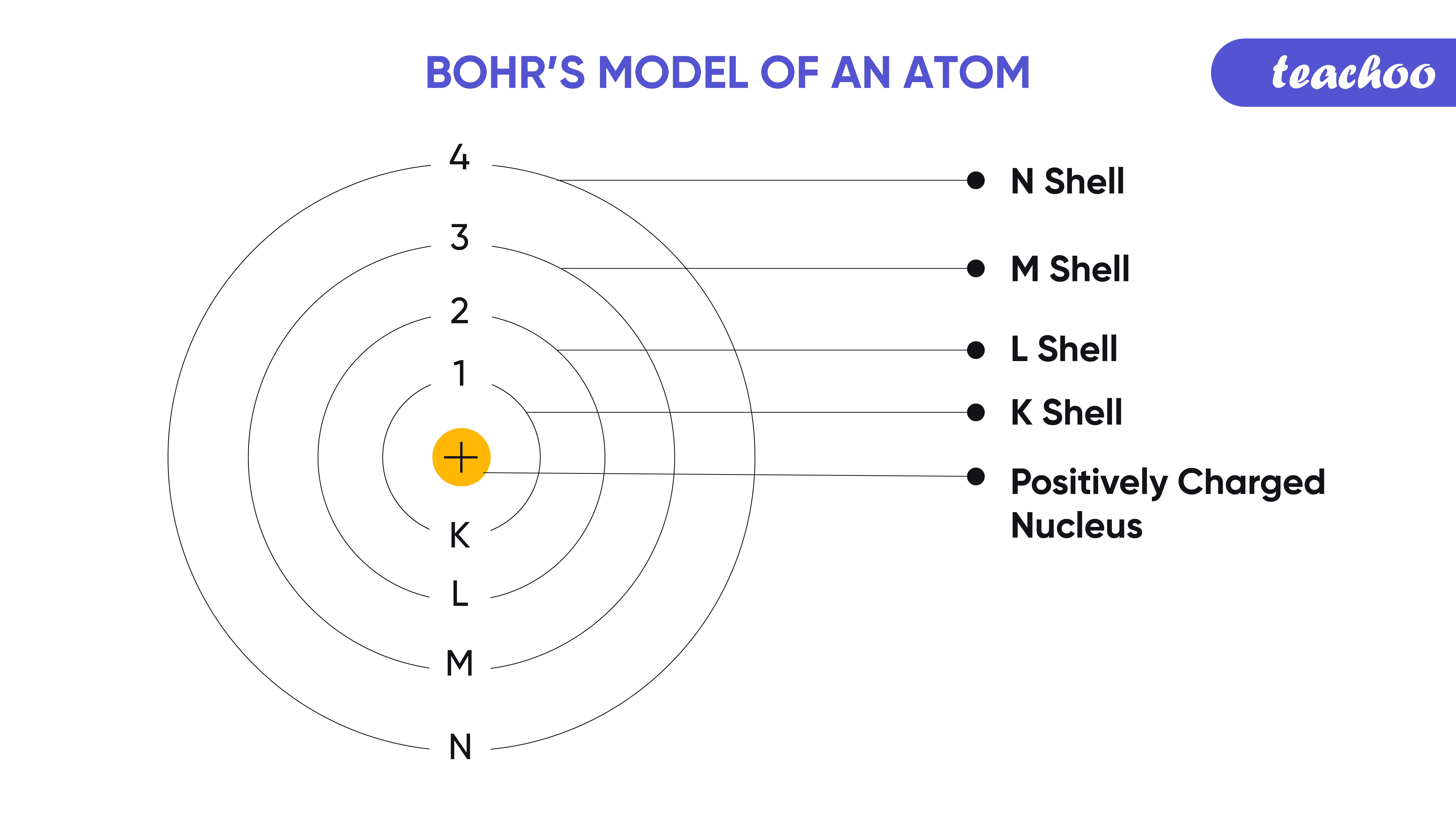
- Electrons Orbit the Nucleus: In Bohr’s model, electrons orbit the nucleus in well-defined energy levels or shells.
- Quantized Energy Levels: Electrons only exist in specific orbits, corresponding to discrete energy levels.
- Quantized Electron Transitions: Electrons can move between orbits by absorbing or emitting photons of light, with energy equal to the difference between the energy levels.
- Spectral Lines: The light emitted or absorbed during electron transitions forms spectral lines, which are unique to each element.
Visualizing Electron Configurations
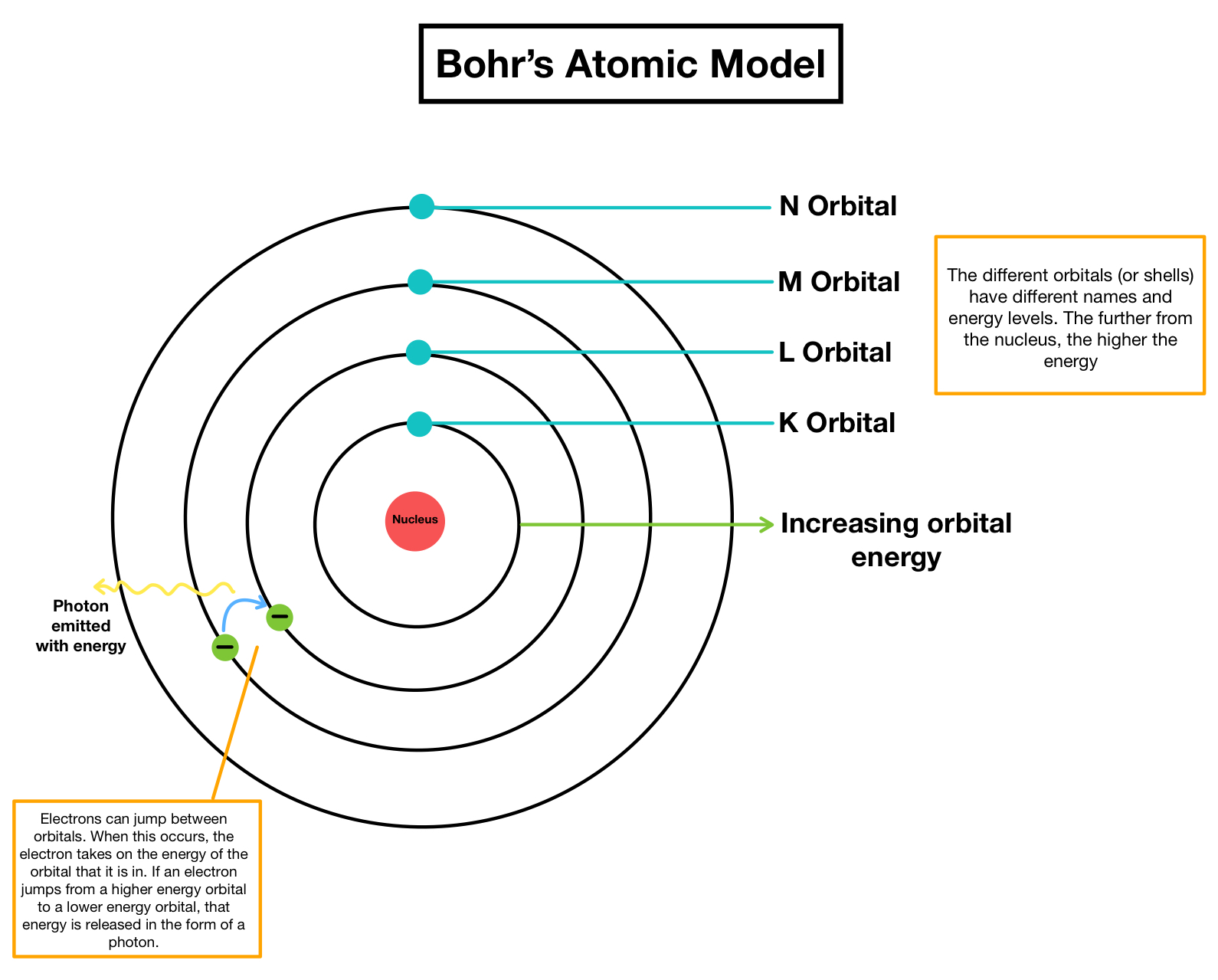
To effectively visualize and understand electron configurations in the Bohr model:
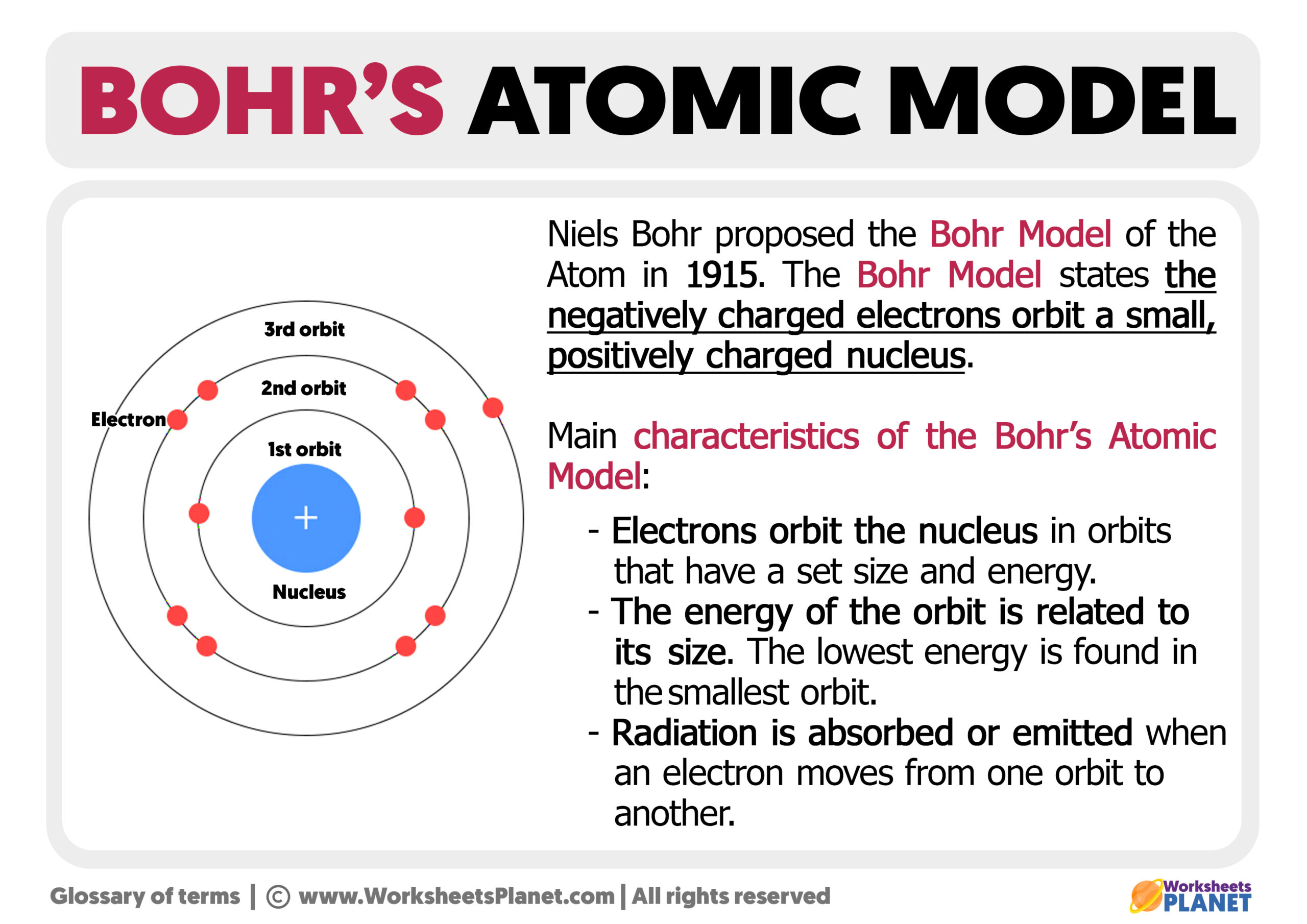
- Identify Energy Levels: Label the energy levels as n=1, n=2, etc., starting from the innermost shell.
- Distribute Electrons: Place electrons in orbits starting from the innermost shell (1s) until the outer shell is filled, following the Aufbau Principle and Hund’s Rule.
- Use Diagrams: Draw or visualize Bohr diagrams to represent electrons as dots or crosses. Use different colors or symbols for different subshells (s, p, d, f).
✏️ Note: Understanding how electrons fill orbits can be complex; diagrams simplify this process and help in visualizing electron behavior.
Mastering Bohr Model Calculations
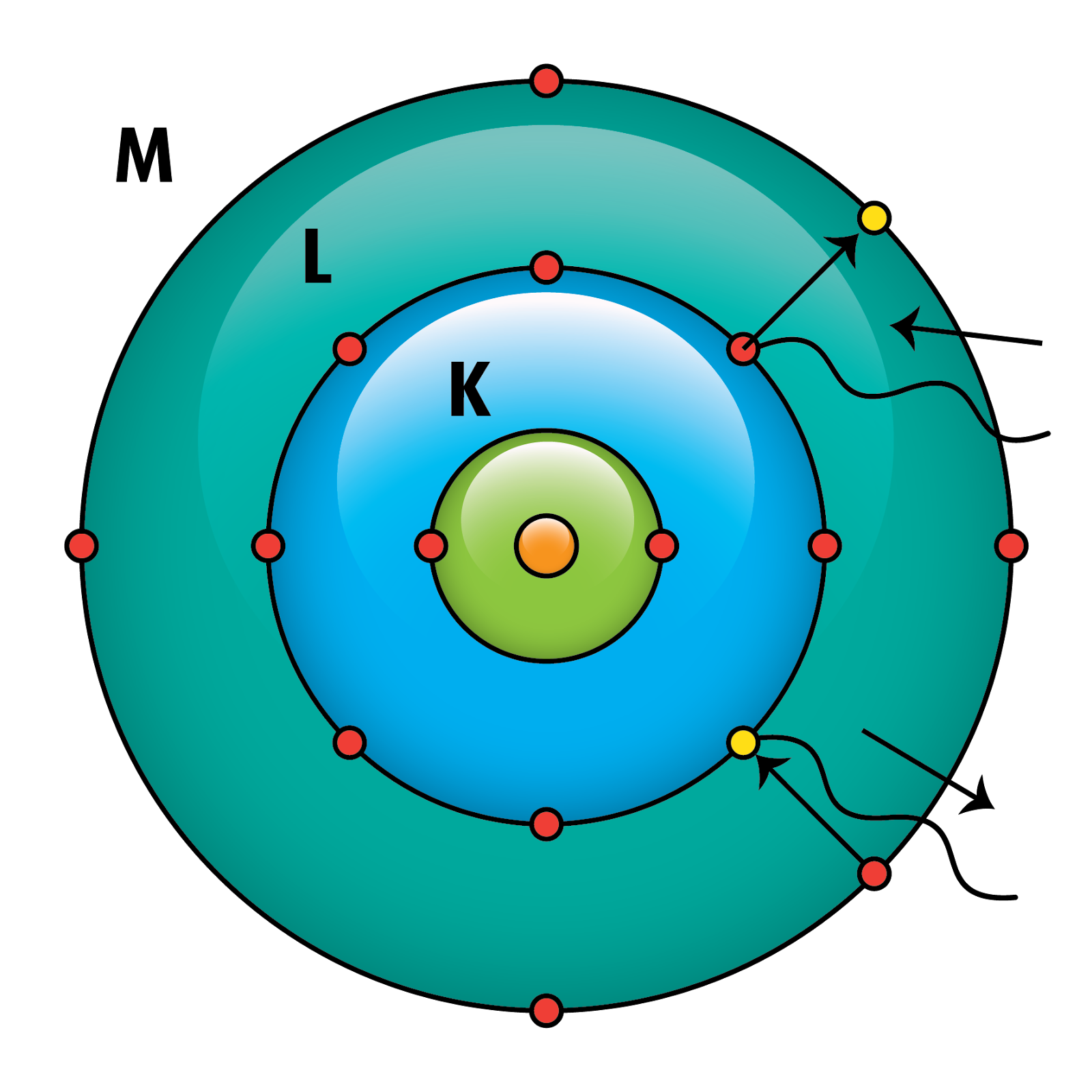
To master calculations related to Bohr’s atomic model:
- Determine Energy Levels: Use the formula for the energy of an electron in a hydrogen atom: [E_n = -\frac{13.6 eV}{n^2}], where (En) is the energy and (n) is the principal quantum number.
- Calculate Energy Transitions: The energy difference when an electron jumps from one level to another can be calculated as: [\Delta E = E{initial} - E_{final}]
- Predict Spectral Lines: Use the Rydberg formula to find wavelengths of emitted light: [\frac{1}{\lambda} = R_H (\frac{1}{n_f^2} - \frac{1}{n_i^2})], where (R_H) is the Rydberg constant, (n_i) and (n_f) are initial and final energy levels, respectively.
Applications of the Bohr Model in Modern Science
The Bohr model, despite its limitations, has significant applications:
- Spectroscopy: Spectral analysis helps in identifying elements in samples, from stars to samples in chemistry labs.
- Atomic Clock: Timekeeping devices use the predictable energy transitions of cesium atoms, inspired by Bohr’s principles.
- X-ray Generation: X-rays are generated when electrons transition from higher to lower energy levels.
- Quantum Mechanics: Bohr’s model paved the way for quantum theory, influencing later models like the Schrödinger equation.
Effective Teaching Strategies for Bohr’s Model
To teach or learn Bohr’s model effectively:
- Use Analogies: Compare atomic orbits to planetary orbits to explain electron behavior.
- Interactive Simulations: Utilize online simulations where students can see electron transitions and spectral emissions in action.
- Build Models: Construct physical models or use kits to visualize electron placement and transitions.
- Real-world Examples: Link Bohr’s model to everyday phenomena like flame tests or fluorescent lighting.
💡 Note: Simulations and real-world examples bring abstract concepts to life, making them more understandable and memorable.
In this comprehensive exploration of Bohr's atomic model, we've covered the essentials of its theory, visualization techniques, practical calculations, modern applications, and educational strategies. By understanding and applying these proven methods, students, educators, and science enthusiasts can enhance their comprehension of atomic structure, paving the way for deeper insights into chemistry and physics. The Bohr model, while simplified, provides a foundational knowledge that is crucial for understanding more complex quantum mechanical models. With this knowledge, we can appreciate how past scientific achievements shape our understanding of the microscopic world, guiding us in future scientific discoveries.
What are the main limitations of the Bohr model?

+
The Bohr model, while groundbreaking, has limitations: it doesn’t account for the wave-particle duality of electrons, cannot explain atomic spectra of elements with more than one electron accurately, and fails to predict electron configurations in multi-electron atoms correctly.
Can Bohr’s model explain all atomic phenomena?

+
No, while it provides a good approximation for hydrogen and some ionized helium, Bohr’s model cannot accurately describe more complex atoms with many electrons or interactions between electrons and the nucleus.
How does the Bohr model relate to modern quantum mechanics?

+
Though simpler, the Bohr model laid the groundwork for quantum mechanics. Its quantized energy levels are reflected in the more sophisticated models of quantum physics, which describe electrons as probability clouds rather than fixed orbits.