Mastering Binary Ionic Compounds: Worksheet Answers

Understanding the Basics of Binary Ionic Compounds

To master binary ionic compounds, understanding their fundamental structure is essential. Ionic compounds consist of ions – charged particles where atoms gain or lose electrons to achieve stable electron configurations, typically from elements on the periodic table's opposite sides.
The Formation of Ionic Bonds

- Metals: Lose electrons to form positive ions (cations).
- Non-metals: Gain electrons to form negative ions (anions).
When these ions are brought together, an ionic bond forms due to the electrostatic attraction between oppositely charged ions, creating a binary ionic compound. This bond is highly directional, leading to a crystal lattice structure where each ion is surrounded by ions of the opposite charge.
Naming Binary Ionic Compounds

There's a systematic approach to naming binary ionic compounds:
Step-by-Step Naming Process

- First element: Use the full name of the metal without any change.
- Second element: Use the base name of the non-metal, with the suffix -ide added to denote the anion.
💡 Note: When the metal has more than one possible charge, use Roman numerals to specify the charge as part of the compound's name.
Worksheet Questions and Answers
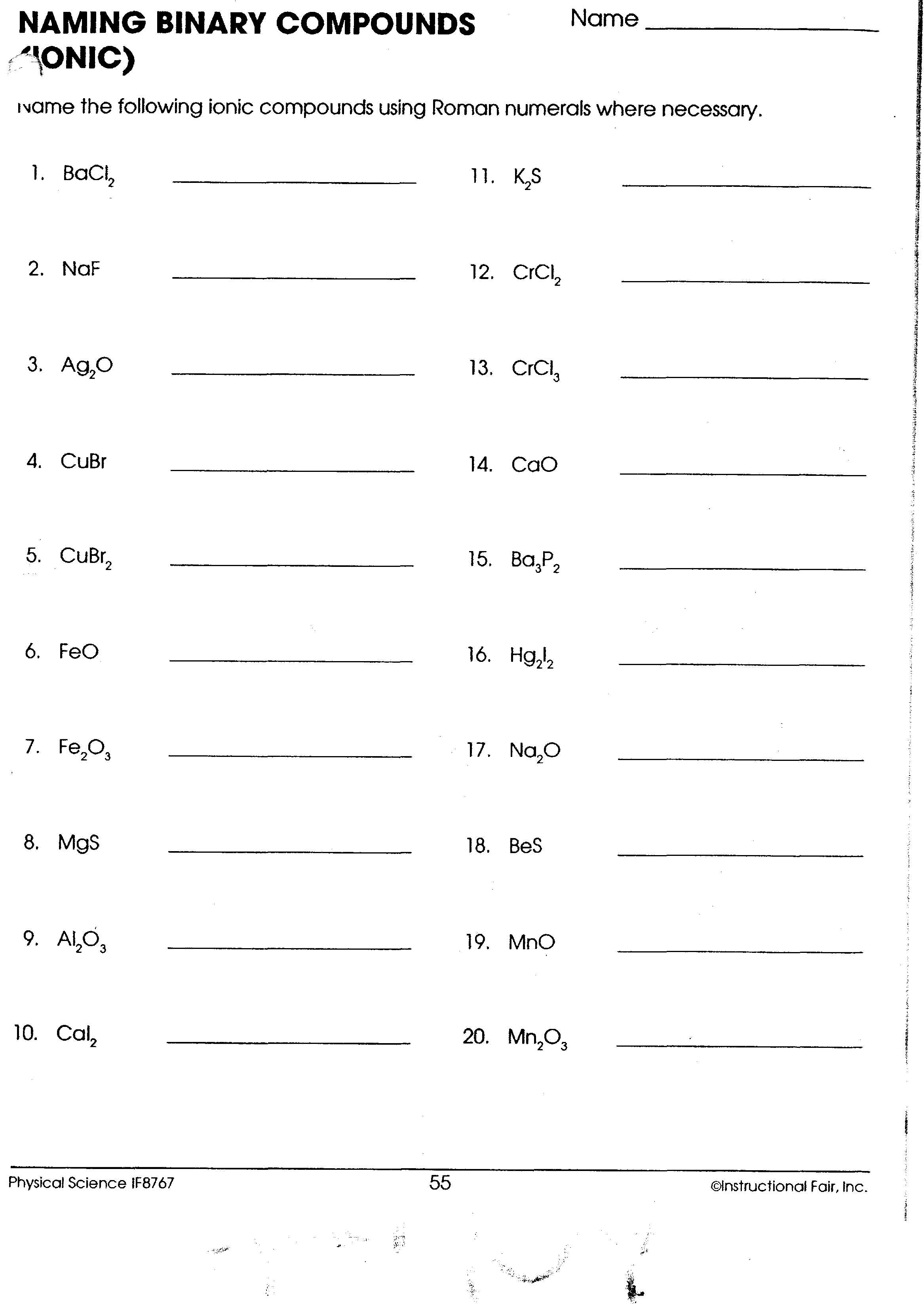
Below are answers to a typical binary ionic compounds worksheet, providing both practice in identifying and naming:
Question 1:

Name the following compounds:
| Formula | Name |
|---|---|
| NaCl | Sodium chloride |
| LiF | Lithium fluoride |
| Fe₂O₃ | Iron(III) oxide |

Question 2:

Write the formula for the following compounds:
| Name | Formula |
|---|---|
| Magnesium nitride | Mg₃N₂ |
| Copper(II) sulfide | CuS |
| Calcium iodide | CaI₂ |
Question 3:
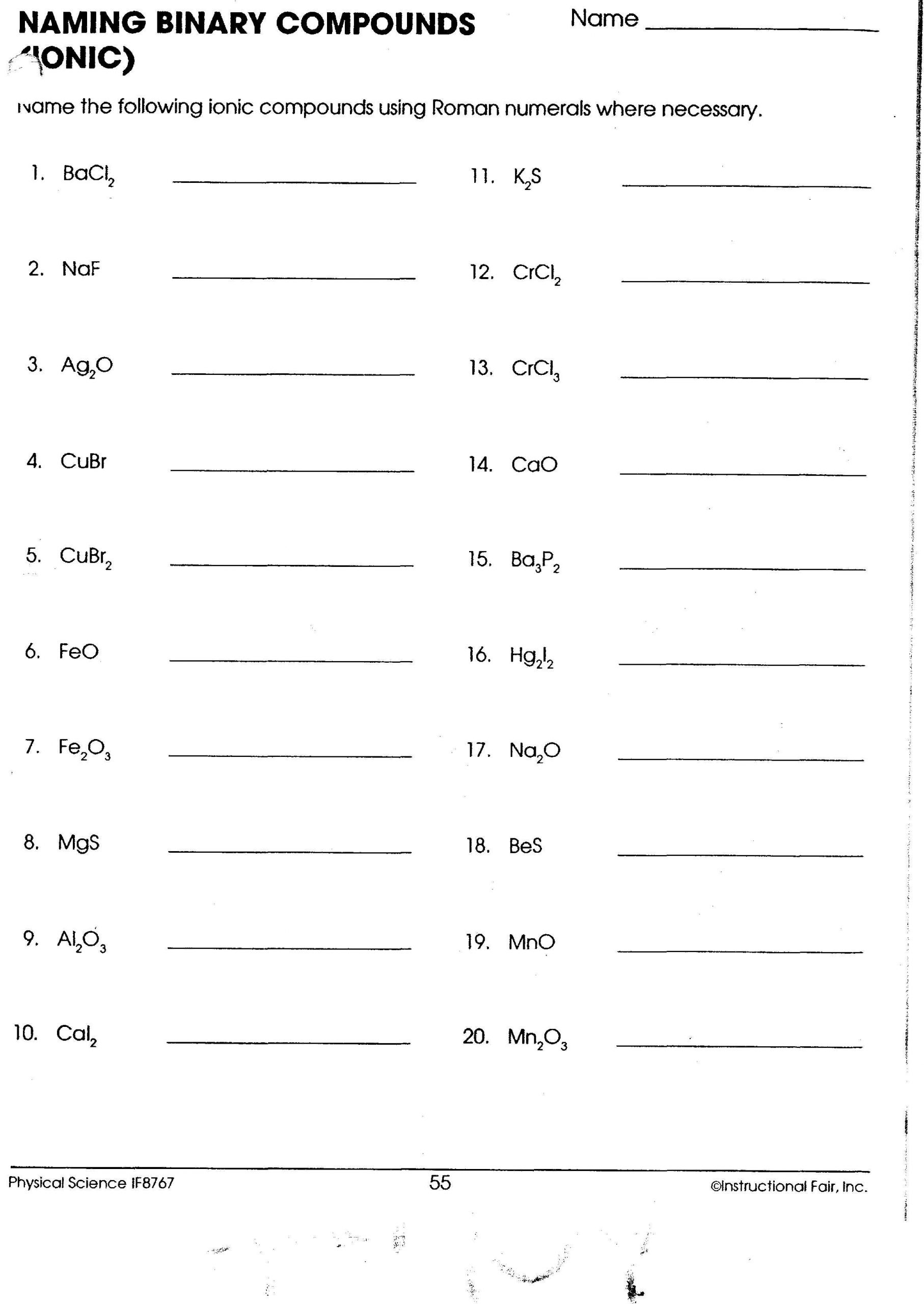
Determine the charge of the cation in each compound:
- CuCl₂: Copper has a +2 charge.
- FeO: Iron has a +2 charge.
- Zn₃P₂: Zinc has a +2 charge.
These examples illustrate how you can determine the charges of elements in binary ionic compounds by balancing the charges to achieve electrical neutrality.
Key Points to Remember

- Ionic compounds form between a metal and a non-metal.
- The charge of each ion determines the formula of the compound.
- Compound names are formed from the names of the ions involved, with the suffix -ide indicating the anion.
💡 Note: When dealing with transition metals, always check their common charges or oxidation states to correctly name compounds.
By understanding these core principles, you'll be well-equipped to identify, name, and predict the properties of binary ionic compounds. This fundamental knowledge not only aids in academic study but also in real-world applications, from understanding chemical reactions in industry to predicting the behavior of minerals and salts in natural environments.
Recapping, the journey into the world of binary ionic compounds has provided us with insights into their formation, naming conventions, and practical applications. This knowledge serves as a cornerstone for further chemical explorations, offering a clear understanding of how ionic interactions shape our chemical world.
What are the differences between binary ionic and covalent compounds?

+
Binary ionic compounds form from a metal and a non-metal where electrons are transferred from the metal to the non-metal, creating ions. Covalent compounds, however, share electrons between non-metal atoms. Ionic bonds are generally stronger, leading to higher melting and boiling points.
Why do some metals need Roman numerals in their compound names?

+
Transition metals and certain main group metals can exist in more than one charge state. Roman numerals are used to indicate which charge the metal ion has in the compound to avoid ambiguity in naming.
Can binary ionic compounds conduct electricity?

+
When in solution (dissolved in water) or melted, binary ionic compounds can conduct electricity due to the mobility of their ions. In solid form, they do not conduct electricity as the ions are fixed in place.