Atomic Structure Worksheet: Answers & Insights
Discovering the intricate details of atomic structure can be both fascinating and complex. Understanding the building blocks of matter is not just a fundamental aspect of chemistry but also forms the basis of numerous scientific disciplines. Whether you are a student, an educator, or someone with a curious mind, this post aims to provide clarity on the fundamental elements of an atom, their arrangement, and how they interact. Here, we will explore answers and insights related to an atomic structure worksheet to deepen your understanding and appreciation of the micro-world of atoms.
Atomic Structure: Basic Components

At the heart of atomic theory lie three primary particles:
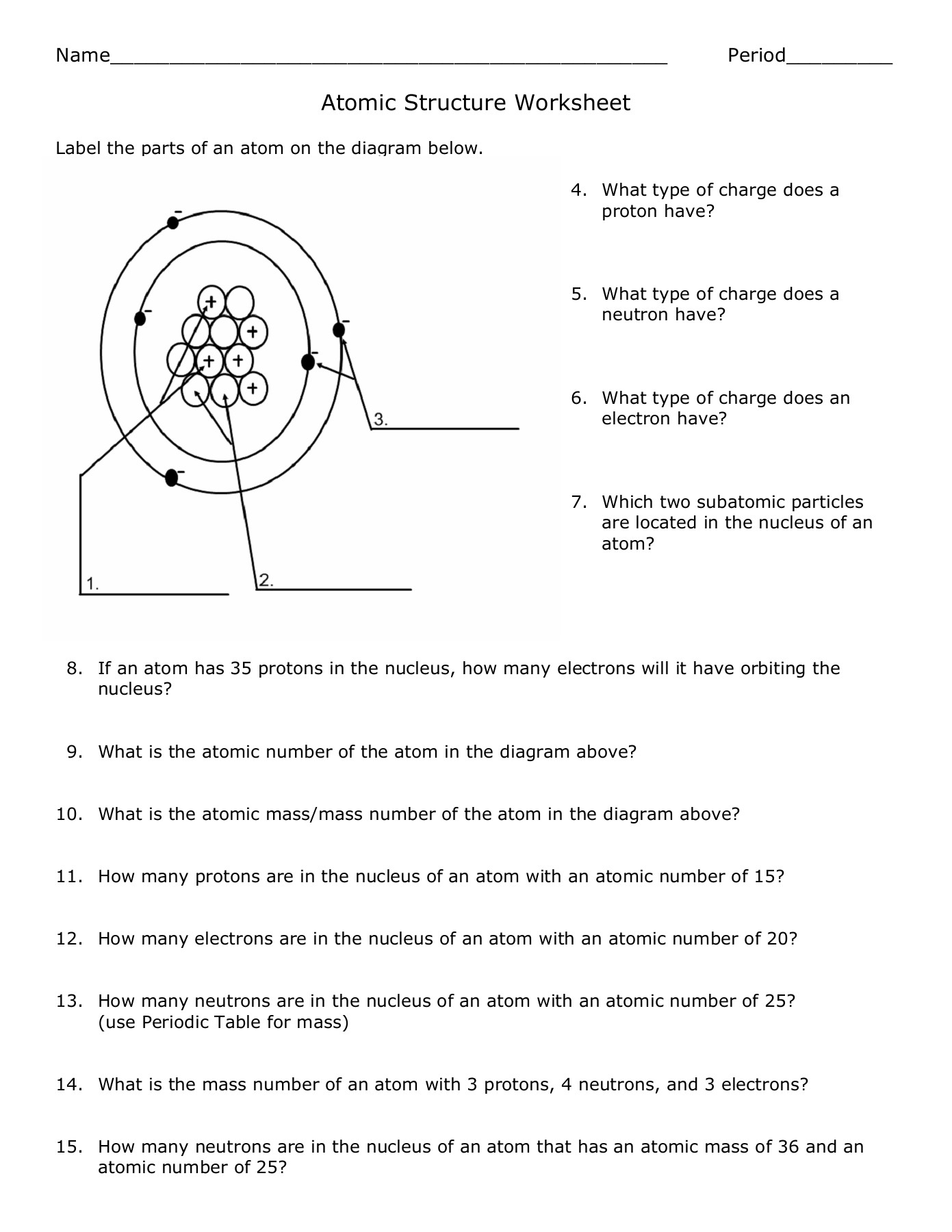
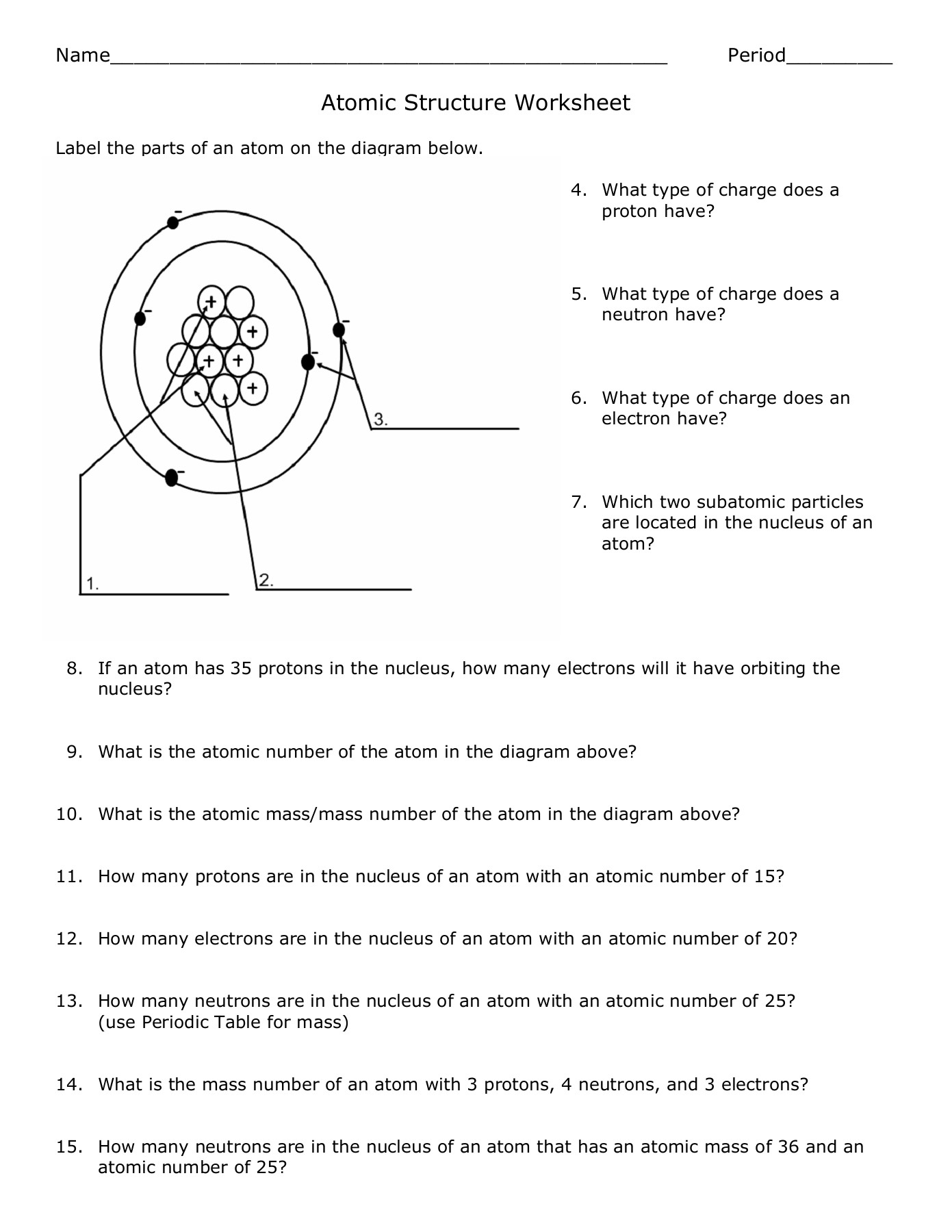
- Protons: Positively charged particles located in the nucleus.
- Neutrons: Neutral particles found in the nucleus, contributing to atomic mass.
- Electrons: Negatively charged particles that orbit the nucleus in distinct energy levels or shells.

Protons and Atomic Number

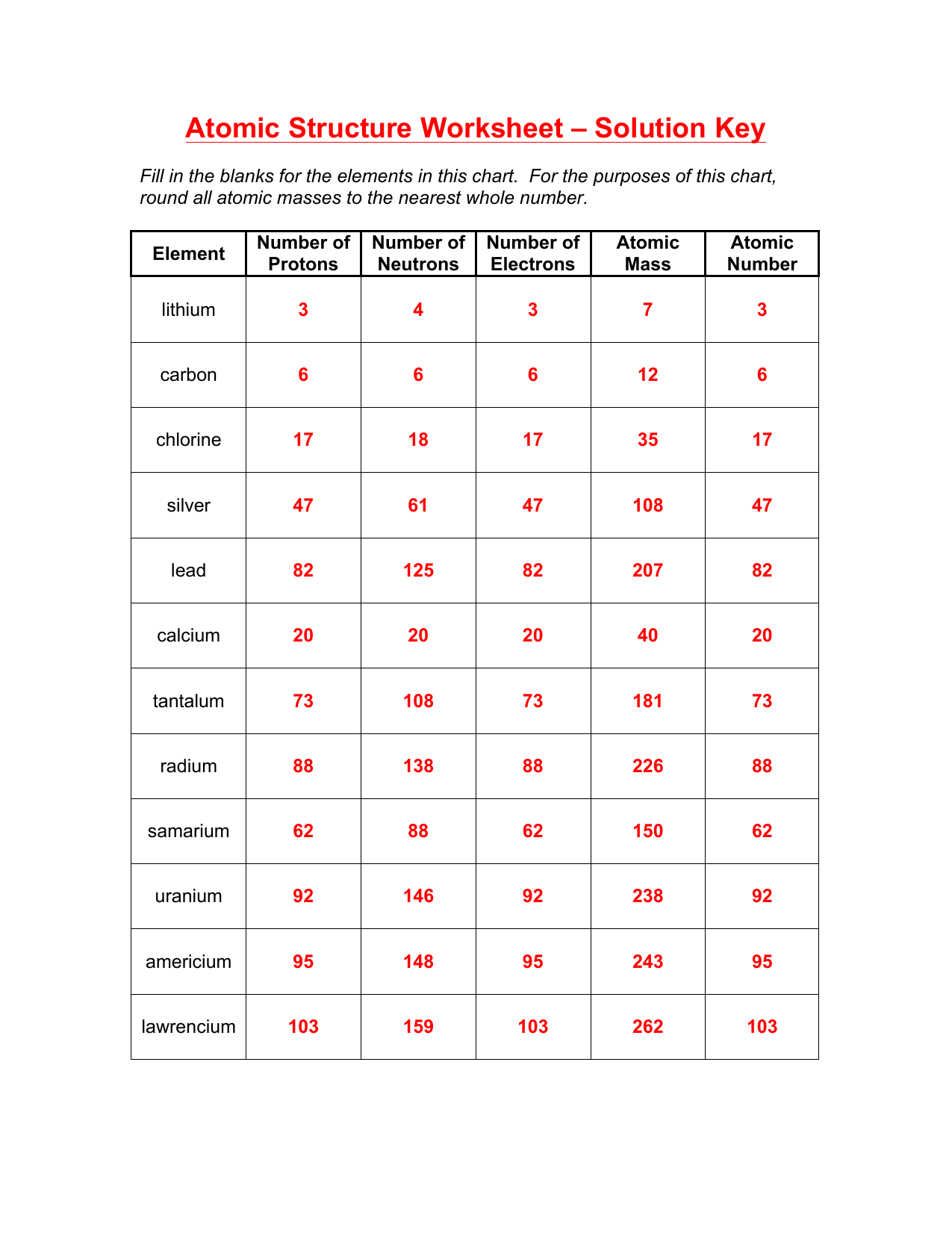
The number of protons in an atom determines its atomic number. This number is unique for each element:
| Element | Atomic Number |
|---|---|
| Hydrogen | 1 |
| Helium | 2 |
| Carbon | 6 |
🌟 Note: The atomic number defines an element, and changing it results in a different element.
Neutrons and Atomic Mass

While protons give us atomic identity, neutrons contribute to an atom’s mass:
- Mass Number = Number of Protons + Number of Neutrons
- Neutrons can vary, leading to isotopes of the same element.
🧠 Note: Isotopes differ in mass but have the same chemical behavior due to identical electron configurations.
Electrons: The Dance Around the Nucleus

Electrons move around the nucleus in shells or energy levels:
- The first shell can accommodate 2 electrons.
- The second shell holds 8 electrons.
- Subsequent shells follow the 2n² rule where n is the shell number.

Delving Deeper: Electron Configurations
Electron configurations are a shorthand way to represent how electrons are organized within an atom. Here’s how to write one:
- Identify the atomic number of the element.
- Fill the electrons into the shells starting from the innermost.
- Represent the configuration in a format like 1s² 2s² 2p⁶.
Here are some examples:
- Hydrogen (H): 1s¹
- Helium (He): 1s²
- Carbon ©: 1s² 2s² 2p²
📌 Note: Electron configurations help explain an element’s chemical behavior and reactivity.
Atomic Models: A Historical Perspective
Models of atomic structure have evolved over time:
- Thomson’s Plum Pudding Model: A positive sphere with electrons embedded.
- Rutherford’s Gold Foil Experiment: Introducing the concept of a dense nucleus.
- Bohr’s Model: Electrons in fixed orbits or energy levels.
- Quantum Mechanical Model: Probability clouds for electron locations.

Ionization and Isotopes

Ions form when atoms lose or gain electrons:
- Cations (Positively charged): More protons than electrons.
- Anions (Negatively charged): More electrons than protons.
Isotopes, as mentioned, are versions of an element with different numbers of neutrons:
- Carbon-12: 6 protons, 6 neutrons.
- Carbon-14: 6 protons, 8 neutrons, used for carbon dating.
Putting Theory into Practice: Atomic Structure Worksheet

Understanding atomic structure often begins with worksheets. Here are some insights and answers commonly found in such exercises:
- Element Identification: Using the periodic table to find atomic number and mass.
- Electron Configuration: Write the configuration for a given element, considering periodic trends.
- Ionic Charges: Determine what type of ion an atom would form based on its position in the periodic table.
- Isotopes: Calculate the average atomic mass given isotopic abundances.
✅ Note: Practice is key to mastering atomic structure. Repetition helps in memorizing element properties.
Summary & Final Thoughts

Delving into atomic structure provides a glimpse into the essence of chemistry and the natural world. By understanding protons, neutrons, and electrons, we unlock the secrets of how elements behave, bond, and form the vast array of substances around us. Here’s a recap of what we’ve learned:
- Protons and atomic numbers define an element’s identity.
- Neutrons add to atomic mass, and their number can vary creating isotopes.
- Electrons, with their energy levels, dictate an atom’s chemistry.
- The evolution of atomic models from simple to complex enhances our understanding.
- Ionization and isotopes add further layers to atomic theory, influencing everything from electricity to radiocarbon dating.
As we continue to unravel the mysteries of the atom, remember that this knowledge is foundational to grasping larger concepts in science and technology. Whether you’re conducting research, developing new materials, or simply appreciating the complexity of the universe, atoms are where it all begins.
What’s the difference between atomic number and mass number?

+
The atomic number indicates the number of protons in an atom’s nucleus, defining the element. The mass number, on the other hand, is the sum of protons and neutrons, representing the atom’s mass.
How do electrons arrange themselves in an atom?

+
Electrons arrange in shells or energy levels. The first shell can hold 2 electrons, the second 8, and subsequent shells can be filled according to the formula 2n² where n is the shell number.
What’s the significance of isotopes in nature?

+
Isotopes play crucial roles in dating geological samples (e.g., carbon dating), medical applications (radioisotopes for diagnostics), and understanding nuclear reactions, influencing everything from climate studies to energy production.