5 Key Facts About Basic Atomic Structure

The study of atomic structure is central to understanding the world of chemistry and physics. When exploring the basics of an atom's makeup, five key facts emerge that are essential for a comprehensive grasp of atomic theory. Let's delve into the details of what constitutes an atom, its fundamental particles, and how these particles interact to give matter its unique properties.
1. Atoms are the Smallest Units of Elements

The concept of an atom as the smallest unit of an element is one of the most foundational principles in chemistry. Here’s what you need to know:
- Each element is made up of one type of atom, distinguished by its atomic number, which indicates the number of protons in the nucleus.
- Atoms cannot be broken down into simpler substances through chemical means, though they can be split or combined during nuclear reactions.
- Isotopes are variations of the same element with the same number of protons but different numbers of neutrons, leading to varying atomic mass.
⚛️ Note: While atoms are considered the smallest units, they are still composed of even smaller subatomic particles.
2. Components of an Atom: Protons, Neutrons, and Electrons
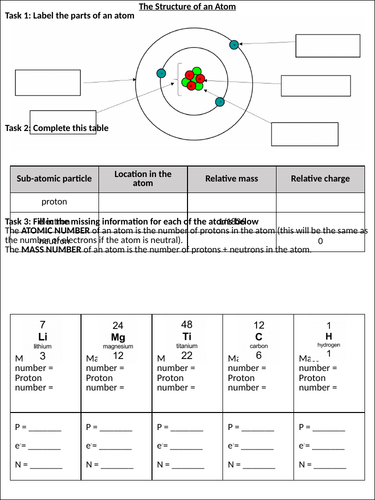
Understanding an atom’s structure involves knowing its constituent parts:
- Protons: Positively charged particles found in the atom’s nucleus. The number of protons determines the element’s atomic number.
- Neutrons: Neutral particles that also reside in the nucleus, contributing to the atom’s mass but not its charge.
- Electrons: Negatively charged particles that orbit the nucleus in energy levels or shells. They play a crucial role in chemical reactions and bonding.
These three particles create the architecture of atoms, and their interplay dictates an element’s chemical behavior.
3. The Nucleus: Heart of the Atom

The nucleus is not just the core of an atom; it’s where most of the atom’s mass is concentrated:
- The nucleus contains protons and neutrons, which collectively make up the mass number of the atom.
- It has a diameter roughly 1⁄100,000th of an atom, but it houses nearly all of its mass.
- Nuclear forces bind protons and neutrons together in the nucleus, counteracting the repulsive forces between protons.
The size and density of the nucleus have significant implications for the stability and reactivity of atoms.
4. Electron Configuration and Energy Levels
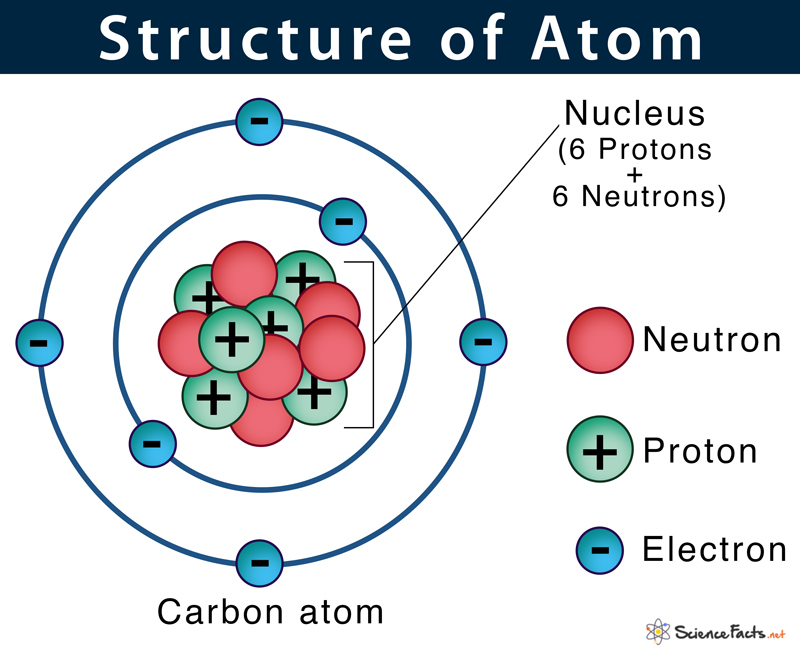
Electrons orbit the nucleus in specific energy levels or shells:
- Each shell has a maximum number of electrons it can hold (2n², where ‘n’ is the shell number).
- Electrons fill the shells from the innermost to the outermost, following the Aufbau principle, Pauli Exclusion Principle, and Hund’s rule.
- The valence electrons, those in the outermost shell, are key to the atom’s chemical properties and how it forms bonds.
Understanding electron configuration is crucial for predicting how atoms will interact and form compounds.
5. Atomic Mass, Atomic Number, and Isotopes

The atomic mass, atomic number, and concept of isotopes are pivotal to atomic structure:
- Atomic Number: The number of protons in the nucleus. Each element has a unique atomic number.
- Atomic Mass: The average mass of an element’s atoms, taking into account the mass of protons, neutrons, and the relative abundance of isotopes.
- Isotopes: Atoms of the same element with different numbers of neutrons. This difference affects atomic mass but not the chemical behavior since the number of protons remains the same.
In summary, the atomic structure is an intricate interplay of subatomic particles. Protons and neutrons in the nucleus provide the mass and identity of the element, while electrons orbiting in energy levels determine its chemical interactions. Knowing these key facts forms a foundation for understanding matter at its most basic level, offering insights into why elements behave the way they do and how they interact to form the substances we see around us.
What makes elements differ from one another?

+
Elements differ primarily by their atomic number, which reflects the number of protons in the nucleus of their atoms. This difference in proton count gives each element its unique properties, affecting how they form bonds and react chemically.
Why are some isotopes radioactive?

+
Some isotopes are radioactive because they have an unstable number of neutrons in relation to the number of protons. This imbalance can lead to nuclear instability, causing the isotope to emit particles or electromagnetic radiation to achieve stability.
How do electrons in the outer shell affect an element’s chemistry?

+
The electrons in the outermost shell, known as valence electrons, are directly involved in chemical bonding. The number of these electrons determines how an atom will bond with others, whether it will gain, lose, or share electrons, and thus defines its reactivity and types of bonds it can form.