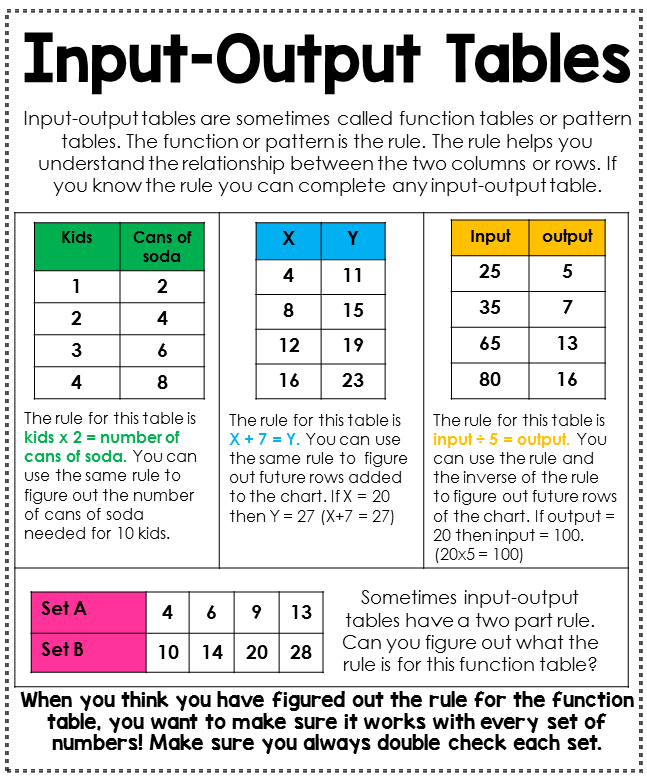
7 Easy Steps to Balancing Nuclear Equations

Balancing nuclear equations is a fundamental skill for anyone delving into the study of nuclear physics or chemistry. These equations detail the transformation of particles during nuclear reactions, which could involve the emission or absorption of particles or a change in atomic nucleus. Here, we will guide you through seven easy steps to make sure your nuclear equations are properly balanced, ensuring you can confidently tackle this aspect of nuclear science.
Step 1: Identify the Reactants and Products

The first step in balancing any chemical or nuclear equation is identifying all the particles involved:
- Reactants - elements or isotopes that undergo nuclear decay or transmutation.
- Products - new elements or isotopes formed, along with any particles or radiation emitted.

🔎 Note: Make sure to verify the atomic numbers and mass numbers to ensure accuracy.
Step 2: Confirm Atomic and Mass Numbers

Each element is defined by its:
- Atomic Number (Z) - the number of protons in the nucleus.
- Mass Number (A) - the sum of protons and neutrons in the nucleus.
These numbers must remain constant throughout the reaction, even if particles or isotopes are being produced.
Step 3: Balance the Atomic Number

Sum the atomic numbers on both sides of the equation:
| Reactants | = | Products |
|---|---|---|
| Atomic number of Reactants | = | Atomic number of Products |

Adjust with appropriate particles if they are not already balanced.
Step 4: Balance the Mass Number

Sum the mass numbers on both sides:
| Reactants | = | Products |
|---|---|---|
| Mass number of Reactants | = | Mass number of Products |
Again, adjust with particles if necessary.
Step 5: Account for Charge Balance

Nuclear reactions often involve the emission or capture of charged particles, so you need to ensure that:
| Sum of Charges of Reactants | = | Sum of Charges of Products |
|---|
Step 6: Adjust Subscripts and Superscripts

Once you have the equation mostly balanced, ensure the atomic and mass numbers are correctly represented:
- Add the necessary particles like electrons (e-), positrons (e+), gamma rays (γ), alpha particles (α), or beta particles (β).
- Verify the positions of superscripts and subscripts for each element or isotope.
⚙️ Note: Remember that subscripts indicate the element's position in the periodic table, while superscripts relate to the mass of the nucleus.
Step 7: Double-Check and Simplify

Lastly, revisit your equation to:
- Reconfirm the sums of atomic numbers and mass numbers.
- Look for any unnecessary particles and remove them if present.
- Simplify the equation where possible.
Balancing nuclear equations can seem daunting at first, but by following these seven easy steps, you can ensure that your equations are accurate and reflective of the nuclear reaction at hand. Each step builds upon the previous one, creating a logical path to ensure balance and consistency in the equation.
Summary

In this post, we've explored the step-by-step process of balancing nuclear equations. From identifying the reactants and products to ensuring the conservation of atomic and mass numbers, charge balance, and correct notation, you've gained the tools necessary to work with nuclear reactions. This knowledge not only allows you to better understand the underlying science but also to communicate your findings clearly.
Why is it important to balance nuclear equations?

+
Balancing nuclear equations is crucial to ensure conservation laws are adhered to, particularly the conservation of mass and energy, which are fundamental in physics.
Can I balance nuclear equations without using particles like electrons or positrons?
+
Yes, but sometimes you will need to include these particles to maintain balance, particularly when dealing with beta decay.
Are there any shortcuts to balance nuclear equations?

+
While there are no universal shortcuts, familiarizing yourself with common nuclear reactions and understanding decay patterns can help streamline the process.
What if my equation has a deficit of mass?

+
This often means you have missed out on a particle or have not accounted for the energy equivalent in the form of radiation or neutrinos.
How do I account for energy in nuclear reactions?

+
Energy changes in nuclear reactions are typically represented by gamma rays (γ) or by the mass difference, where a decrease in mass results in an increase in energy according to Einstein’s famous E = mc².