5 Expert Tips for Balancing Chemistry Equations Easily

Understanding the Basics of Chemical Equations

Before diving into the intricacies of balancing chemical equations, it’s crucial to understand what they represent. A chemical equation is a symbolic representation of a chemical reaction, where reactants are converted into products. The reactants are substances that start the reaction, while the products are the substances formed as a result of the reaction.
Balancing these equations is essential because it adheres to the Law of Conservation of Mass, which states that matter cannot be created or destroyed in a chemical reaction. This means the number of atoms for each element must be the same on both sides of the equation. Here's how you can ensure this:
- Identify all atoms present in each molecule on both sides of the equation.
- Count how many atoms of each element exist before and after the reaction.
- Use coefficients (numbers placed in front of formulas) to adjust the number of molecules, not subscripts which alter the formula itself.
Tip 1: Use an Inventory System

One of the most effective ways to balance chemical equations is by keeping an inventory of the atoms. Here’s how you do it:
- Count the Atoms: Write down the number of atoms for each element on both sides of the equation.
- Balance One Element at a Time: Start with elements that appear in only one compound on each side of the equation. Adjust coefficients to balance that element.
- Check and Adjust: After balancing one element, check the equation again. Sometimes, balancing one element can unbalance others.
- Repeat the Process: Continue balancing until every element is in balance.
⚠️ Note: Remember, changing subscripts in chemical formulas is not allowed. You can only change coefficients.
Tip 2: Utilize the Least Common Multiple

When you encounter fractions or decimals while balancing an equation, find the least common multiple (LCM) of the coefficients. This approach ensures that all the coefficients are whole numbers, making the equation more readable and easier to work with.
- If you end up with an equation like this: 1.5A + B → C, multiply the entire equation by 2 to get 3A + 2B → 2C.
Tip 3: Apply a Systematic Approach

A systematic method like Balancing by Inspection can be employed, especially when dealing with more complex reactions:
- Select Elements to Balance First: Start with metals, then non-metals, hydrogen, and oxygen (in that order).
- Balance Gases Last: Since gases like O2 and H2 often appear in equations with whole number coefficients, balance these last.
- Check Charges: If ionic compounds are involved, ensure the charges balance as well.
✏️ Note: When balancing equations for redox reactions, also ensure electron transfer is balanced.
Tip 4: Simplify Your Coefficients

After balancing an equation, you might find yourself with large, unwieldy coefficients. Simplify the equation by:
- Finding the greatest common divisor (GCD) of all coefficients.
- Dividing all coefficients by this GCD to simplify the equation.
Tip 5: Practice with Examples

Practice is the key to mastering the art of balancing chemical equations. Here are some examples to illustrate the tips discussed:
| Original Equation | Balanced Equation |
|---|---|
| Na + H2O → NaOH + H2 | 2Na + 2H2O → 2NaOH + H2 |
| C6H12O6 + O2 → CO2 + H2O | C6H12O6 + 6O2 → 6CO2 + 6H2O |
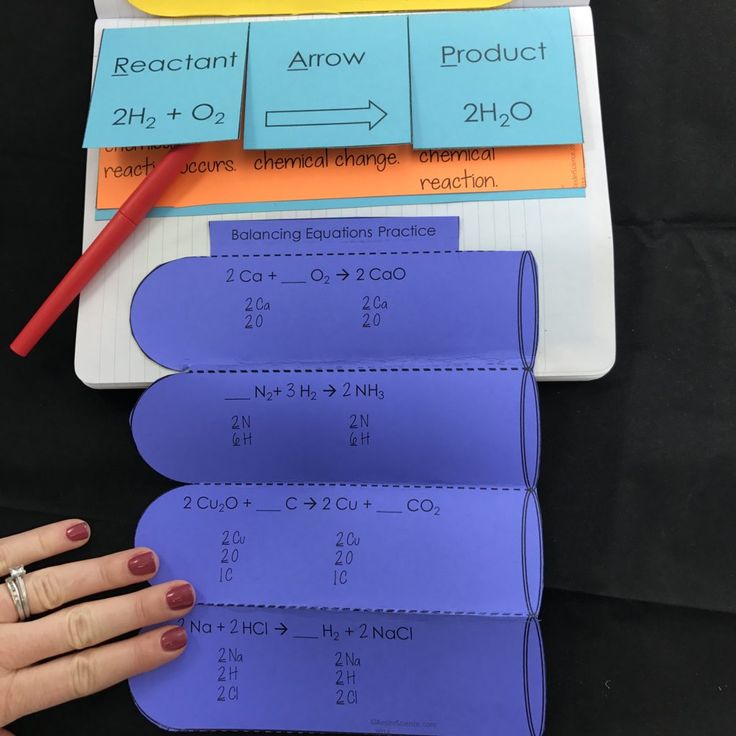
🔍 Note: Remember, the coefficients you add change the quantity of the substances, not their identity or the molecular structure.
To summarize, mastering the balancing of chemical equations involves:
- Understanding the basic principles of chemical reactions.
- Employing systematic methods like inventory systems and balancing by inspection.
- Using mathematical concepts like LCM and GCD for simplification.
- Practicing with various types of reactions to refine your technique.
Balancing chemical equations might seem daunting at first, but with these expert tips, the process becomes manageable and even enjoyable. The key is to maintain the integrity of the chemical reaction while adjusting quantities to match the law of conservation of mass. Whether you are a student, educator, or professional chemist, these tips will streamline your work with chemical equations.
Why is it important to balance chemical equations?

+
Balancing ensures the law of conservation of mass is not violated, providing an accurate representation of the reaction occurring.
Can you balance equations with decimal or fractional coefficients?

+
While you can balance equations with decimals or fractions, it’s typically more convenient to use whole numbers by multiplying through by the smallest factor that removes the fractions.
What should I do if an element is present only in one molecule on either side?

+
Start with those elements first because changing their coefficients will not immediately unbalance other elements in the equation.



