10 Quick Answers to Balance Chemical Equations

Balancing chemical equations is a fundamental skill in chemistry, crucial for understanding chemical reactions and predicting outcomes. For students and professionals alike, this process can seem daunting at first. However, with a structured approach and some practical examples, balancing equations becomes more manageable. In this post, we'll walk through 10 quick answers to common questions about balancing chemical equations, helping you master this essential aspect of chemistry.
Why is Balancing Chemical Equations Important?
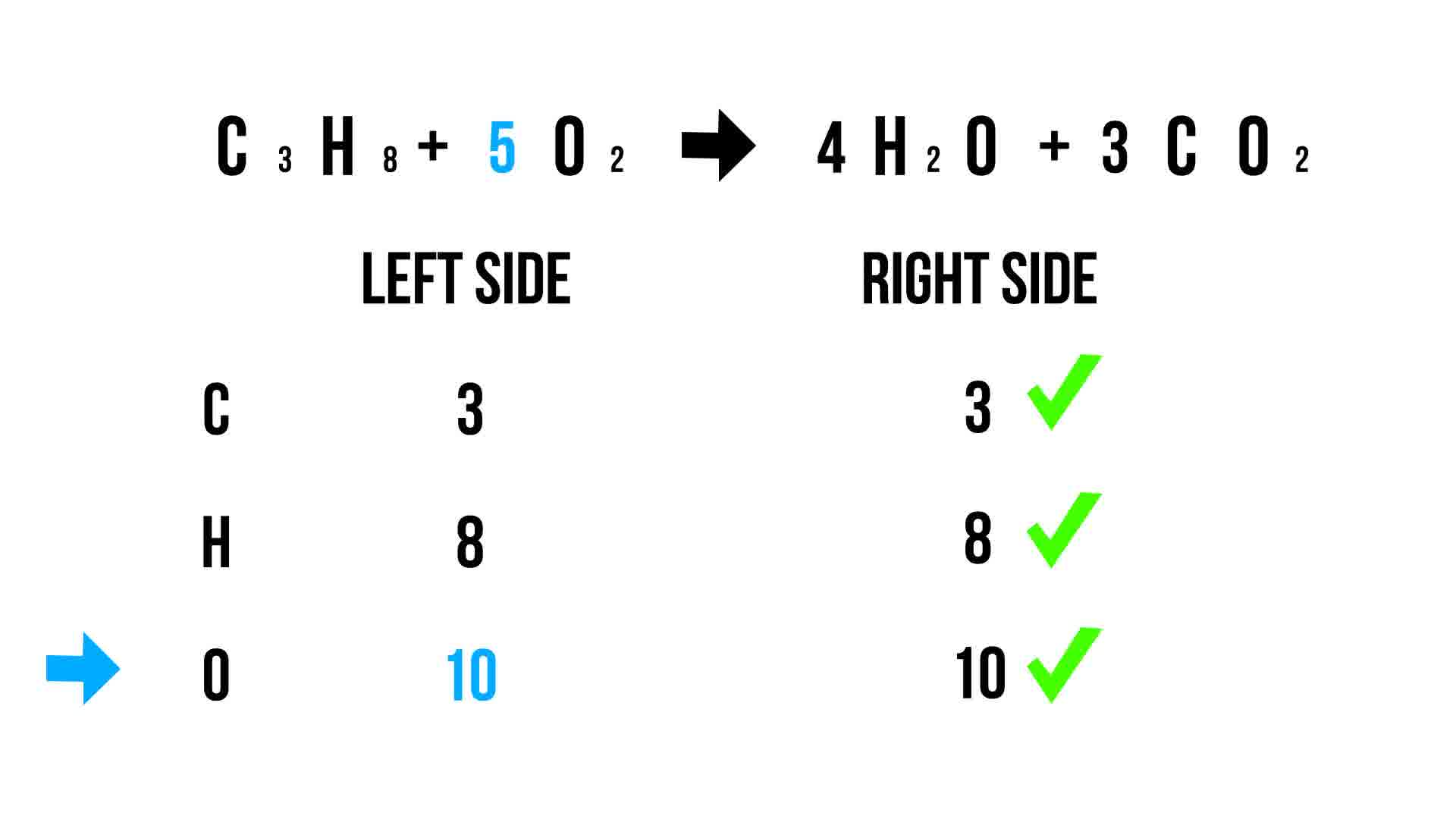
Balancing chemical equations ensures that the law of conservation of mass is obeyed. This law states that mass is neither created nor destroyed during a chemical reaction. Here’s why this matters:
- It reflects the real chemical changes that take place, making calculations about the amount of substances involved precise.
- It allows us to determine the stoichiometry of a reaction, which is crucial for predicting how much product can be formed from given reactants.
- It aids in understanding reaction mechanisms and the efficiency of the reaction process.
What are the Basic Steps to Balance an Equation?

The process of balancing a chemical equation can be broken down into these steps:
- Write down the chemical reaction. Start with the correct formulas for all reactants and products.
- Count the number of atoms for each element. This helps in knowing what needs to be balanced.
- Choose an element to balance first. Typically, start with metals, then non-metals, hydrogen, and oxygen last.
- Add coefficients to balance atoms. Use the smallest whole numbers to balance the equation, adjusting coefficients until both sides have the same number of atoms for each element.
- Check your work. Ensure all atoms are balanced and that no coefficients are improperly used or missing.
What Happens if the Coefficients are not Whole Numbers?

If your initial attempt at balancing results in fractional coefficients, you can simply multiply the entire equation by the least common multiple (LCM) of the denominators to clear the fractions:
- Example: If you have an equation like ( \frac{1}{2}O_2 + H_2 \rightarrow H_2O ), multiply everything by 2 to get ( O_2 + 2H_2 \rightarrow 2H_2O ).
💡 Note: Fractional coefficients are acceptable in theoretical calculations but are typically avoided in practical chemical scenarios to ensure you are working with tangible quantities.
Can You Use Empirical Formulas to Balance Equations?

While empirical formulas show the simplest whole number ratio of elements in a compound, they are not typically used for balancing equations because they do not provide the correct molecular structure. Instead:
- Ensure that you are using the correct molecular formula in your chemical equation to represent the actual compounds involved.
What if an Element Appears in Multiple Compounds?

When an element appears more than once on a single side of the equation, follow this strategy:
- Balance the element involved in the most compounds first, then move on to the other elements systematically.
- Example: For the equation ( Fe + H_2O \rightarrow Fe_3O_4 + H_2 ):
Element Reactants Products Fe 1 3 O 1 4 H 2 2 
You would balance iron first by putting a coefficient of 3 in front of Fe on the reactant side, making it ( 3Fe + H_2O \rightarrow Fe_3O_4 + H_2 ).
How Do You Handle Redox Reactions?

Balancing redox (reduction-oxidation) reactions often involves:
- Assigning oxidation numbers.
- Balancing atoms undergoing change in oxidation states.
- Balancing the rest of the atoms.
This requires a more detailed approach and might involve using the half-reaction method:
- Divide the reaction into half reactions.
- Balance each half-reaction separately.
- Combine the half-reactions, canceling out common ions or atoms.
- Add water (H₂O) or H⁺ ions as necessary to balance charges and oxygen atoms.
Do I Need to Balance Ions?

Ionic compounds in a chemical equation should be balanced like any other molecule. Here are some considerations:
- Balance the charge. If the equation involves ions, ensure that the charge on both sides is equal.
- Spectator ions. These ions do not participate in the reaction and can be excluded from the net ionic equation after balancing.
🔍 Note: Understanding the role of spectator ions is crucial for balancing equations involving precipitation reactions.
What if a Reaction Produces Gas?

When a reaction produces a gas, consider:
- The gas might need to be balanced individually from other elements.
- Sometimes, the gas might be part of a decomposition reaction, where balancing the number of atoms for the gas molecule is key.
Can Balancing Chemical Equations Help in Problem-Solving?

Yes, balancing chemical equations is not just an academic exercise:
- It helps in limiting reactant problems where you need to calculate how much of each reactant is required.
- It’s essential for stoichiometry, calculating the theoretical yield and efficiency of reactions.
- Balanced equations are fundamental in many chemical analyses and experiments for accurate prediction and observation.
Final Reflections

Understanding how to balance chemical equations is not just about getting the right numbers on both sides of an equation. It’s about comprehending the essence of chemical interactions, the movement of atoms, and the conservation of matter. Each step in the process reflects a real chemical occurrence, making this skill invaluable in both laboratory settings and theoretical studies. With these ten answers in your toolkit, you’ll be better equipped to tackle any balancing equation problem that comes your way, enhancing your chemical intuition and analytical prowess.
What is a Chemical Equation?

+
A chemical equation represents a chemical reaction using chemical formulas. It shows the reactants, products, and the relative amount of each involved in the reaction.
Why Can’t I Balance Equations by Changing Subscripts?

+
Subscripts in chemical formulas indicate the number of atoms of each element within a molecule. Changing these would mean you are altering the compound itself, which is not chemically accurate.
How Does Balancing Affect Equilibrium?

+
Balancing equations does not directly affect the equilibrium of a reaction, but it provides the correct proportions of reactants and products at equilibrium, ensuring mass balance.
What to do if the equation doesn’t seem to balance easily?

+
If the equation is hard to balance, it might require you to double-check the initial reaction for accuracy. Alternatively, use trial and error, consider redox balancing, or look for known similar reactions for comparison.
Can software help in balancing chemical equations?

+
Yes, there are numerous online tools and software programs that can balance equations for you. However, understanding the manual process is crucial for learning and scientific literacy.