5 Essential Steps to Master Balancing Chemical Equations

Balancing chemical equations is not just an essential skill for students of chemistry; it's fundamental for anyone interested in understanding chemical reactions at a basic level. Whether you're preparing for an exam, a career in the sciences, or just satisfy your curiosity, mastering the art of balancing chemical equations can be profoundly rewarding. Here, we'll explore the five critical steps to become proficient in this key aspect of chemistry.
1. Understand the Basics of Chemical Equations

Before diving into balancing, you need to grasp what a chemical equation represents. A chemical equation is a symbolic representation of a chemical reaction where the reactants are shown on the left side and the products on the right. Each element or compound involved is represented by its chemical formula, and the equation must be balanced to reflect the law of conservation of mass.
- Reactants - Chemicals before the reaction occurs.
- Products - Chemicals formed after the reaction.
- Equal Mass - The mass of reactants must equal the mass of products.
💡 Note: Always check your work by ensuring the total number of atoms of each element is the same on both sides of the equation.
2. Identify the Elements and Write the Skeletal Equation

Start by writing down the chemical formulas of all reactants and products. This step is often referred to as the skeletal equation. Here, the focus is on:
- Getting all the atoms' symbols correct.
- Ensuring the charge balance for ionic species.
For example, consider the reaction of hydrogen and oxygen to form water:
| H2 | + | O2 | → | H2O |

3. Count the Number of Atoms

Once you have your skeletal equation, tally up the number of atoms for each element on both sides. This step is crucial because it helps you identify where imbalances exist:
- Write down the count for each element in the reactants and products.
Example: For the above equation, we have:
- Hydrogen: 2 H on the left, 2 H on the right.
- Oxygen: 2 O on the left, 1 O on the right.
4. Use Coefficients to Balance the Equation
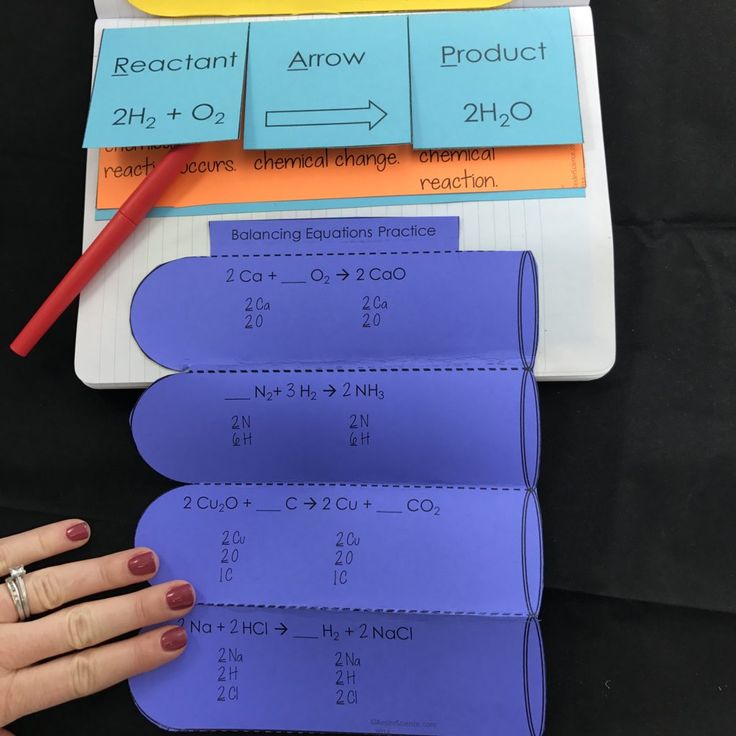
Now, apply the coefficients in front of each chemical formula to balance the equation. The goal is to ensure that the number of atoms of each element is equal on both sides. Here are some tips:
- Start with elements that appear in only one reactant and one product.
- Save elements like hydrogen and oxygen for last as they often appear in many compounds.
Continuing with our example:
- To balance the oxygen, we add a coefficient of 2 in front of water: H2 + O2 → 2H2O.
- Now we have:
- Hydrogen: 2 H on the left, 4 H on the right.
- Oxygen: 2 O on the left, 2 O on the right.
To balance the hydrogen, we adjust the coefficient of hydrogen:
- H2 + O2 → 2H2O becomes 2H2 + O2 → 2H2O.
Now the equation is balanced:
- Hydrogen: 4 H on the left, 4 H on the right.
- Oxygen: 2 O on the left, 2 O on the right.
5. Verify Your Work

Double-check your work to ensure every element’s atoms balance perfectly. Sometimes, you might need to rebalance or make small adjustments. Here are some key points to remember:
- Ensure all elements are balanced.
- Check for the simplest ratio of coefficients to avoid unnecessary complexity.
- If the equation involves polyatomic ions, treat them as single units if they remain unchanged in the reaction.
🔎 Note: If you find that balancing by inspection doesn't work, consider using algebra or a systematic approach to solve for the coefficients.
By following these steps diligently, you'll not only understand how to balance chemical equations but also gain a deeper appreciation for chemical reactions and the law of conservation of mass.
This journey into balancing equations teaches you not just about the math but also about the chemical interactions at a molecular level. It's a skill that not only applies to academic chemistry but also to practical applications in fields like environmental science, pharmacy, and materials engineering.
Why is it important to balance chemical equations?

+
Balancing chemical equations ensures that the mass of substances is conserved in accordance with the law of conservation of mass. This principle states that matter cannot be created or destroyed during a chemical reaction, thus the number and type of atoms on each side of the equation must be the same.
Can I balance any chemical equation by just using coefficients?

+
Yes, for most simple chemical reactions, you can balance the equation solely by adjusting coefficients. However, for more complex reactions involving redox processes or half-reactions, additional steps or methods might be required.
What should I do if I can’t balance an equation?

+
If balancing by inspection is challenging, try using algebra or systematic methods like the half-reaction method or algebraic balancing to find the correct coefficients. If you still struggle, double-check the chemical formulas to ensure they are correct.



