7 Proven Techniques for Balancing Chemical Equations

Balancing chemical equations is a fundamental skill in chemistry, essential for understanding reactions, stoichiometry, and more. It's not just about matching numbers but understanding the conservation of mass and energy. Here, we'll explore seven proven techniques to help you balance equations effectively.
1. The Law of Conservation of Mass

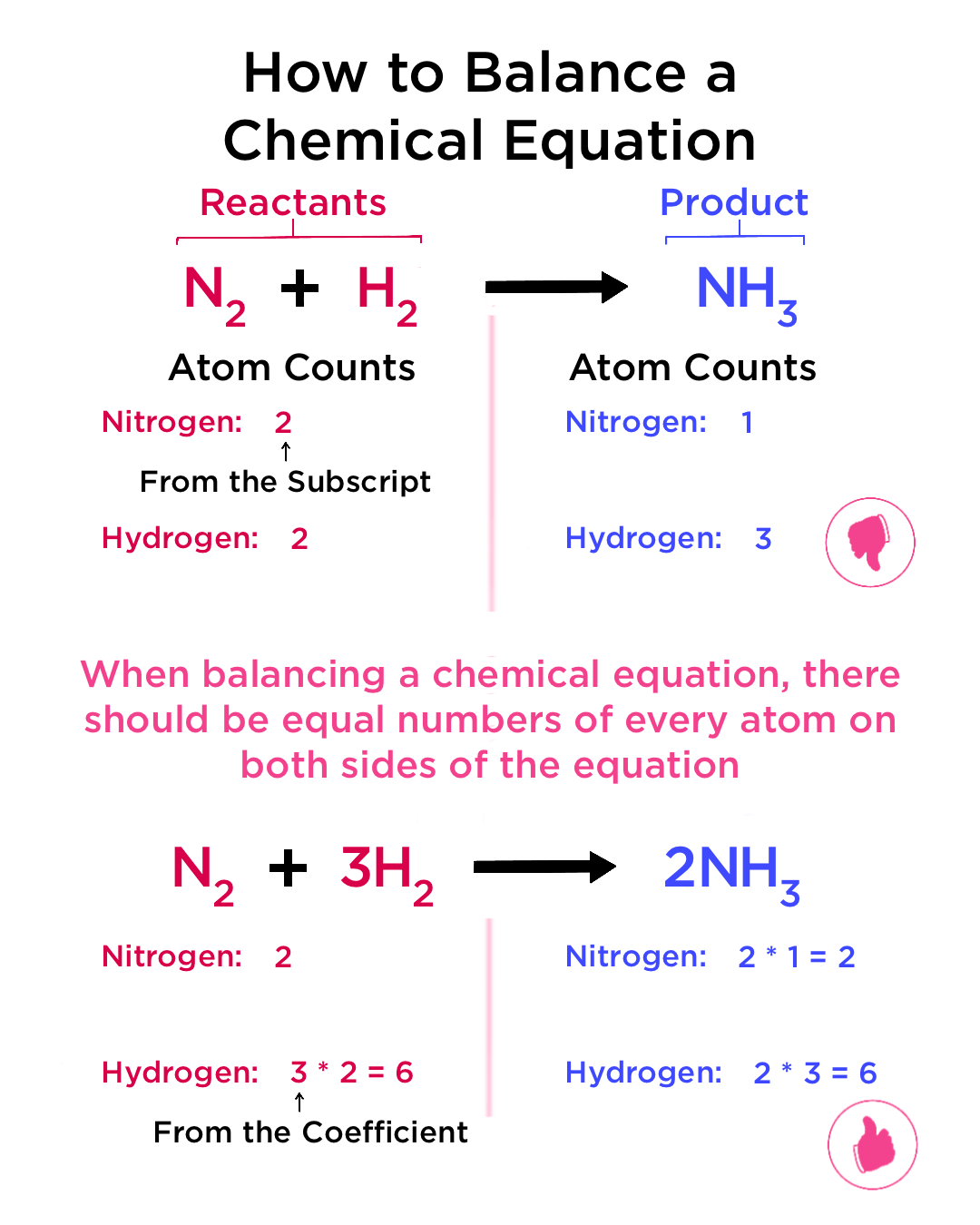
At the heart of balancing chemical equations lies the Law of Conservation of Mass, which states that mass is neither created nor destroyed during a chemical reaction. This means the number of atoms of each element on the reactant side must equal the number on the product side.
- Ensure you count all the atoms.
- Start with the most complex molecule or the one with the highest number of elements.
Example:
When balancing the equation for the combustion of methane: CH4 + O2 → CO2 + H2O
💡 Note: You can balance oxygen and hydrogen by manipulating the coefficients to make sure the equation obeys the law of conservation of mass.
2. Inspection Method

Inspection, or trial and error, is the simplest technique to start with when you're learning to balance equations. Here's how you proceed:
- Write the skeleton equation, ensuring all chemical species are correct.
- Start with elements that appear only once on each side.
- Adjust coefficients sequentially to achieve balance.
This method works well for simple reactions but can become cumbersome for more complex systems.
3. Algebraic Method

When equations become more complex, the algebraic method can provide a systematic approach:
- Assign unknown coefficients to each chemical species (x, y, z, etc.).
- Write down the equations where each element's total number of atoms must equalize between reactants and products.
- Solve these equations algebraically to find the coefficients.
This technique is particularly useful for reactions with polyatomic ions or when the Inspection method fails.
📝 Note: While algebra is involved, you don't need to solve complex equations for every reaction, just those where inspection doesn't lead to quick results.
4. Ion-Electron Method for Redox Reactions

Balancing redox reactions involves additional steps due to electron transfers. Here’s the approach:
- Identify the oxidation and reduction: Assign oxidation numbers to each atom.
- Separate into half-reactions: Write out the oxidation and reduction half-reactions.
- Balance the atoms other than O and H: Using stoichiometry, balance atoms other than O and H.
- Balance O and H: Use H2O and H+ (or OH- in basic solution).
- Balance the charge: Use electrons (e-) to equalize the charges in each half-reaction.
- Multiply to balance electron transfer: Ensure the same number of electrons is lost by one species as gained by another.
- Add half-reactions: Combine them into a balanced full equation.
5. Half-Reaction Method

Similar to the Ion-Electron Method, but less focused on redox specifics, this method can be used for any chemical reaction:
- Write the reaction as two half-reactions.
- Balance atoms.
- Balance charge using electrons.
- Balance electrons between half-reactions.
- Add the balanced half-reactions.
6. Matrix Method

More advanced, this method involves:
- Constructing a matrix where rows represent elements and columns represent compounds.
- Using matrix algebra to solve for coefficients.
It's a powerful tool for very complex reactions but requires understanding of linear algebra.
7. Iterative Method (Simulation)

An iterative or simulation approach involves:
- Simulating reactions at the molecular level to see how atoms come together.
- Using software or online tools to visualize this process.
- Adjusting the simulation to match the observable outcomes.
This method provides insight into the dynamic nature of chemical reactions but is more theoretical and used in advanced research rather than everyday equation balancing.
Summary of Key Points

In this exploration of balancing chemical equations, we've covered various techniques from the simplest visual inspection to sophisticated algebraic and matrix methods. The choice of method depends on:
- The complexity of the reaction.
- The learner's mathematical aptitude.
- The available resources and tools.
Balancing chemical equations isn't just about number crunching; it's a reflection of how nature conserves mass and energy, making chemistry not just a science but a puzzle to be solved with each reaction.
Why is balancing chemical equations important?

+
Balancing chemical equations is crucial for understanding the stoichiometry of a reaction, predicting yields, and ensuring that mass and energy are conserved during the process.
Which method is best for beginners?

+
The Inspection Method is generally the easiest for beginners due to its straightforward visual approach to balancing simple equations.
Can these methods be applied to any chemical reaction?

+
Yes, any chemical reaction can be balanced using one of the techniques discussed, although some might require more advanced methods like matrix or iterative simulation for very complex reactions.
How can software or online tools help with balancing?
+
Software and online tools can simulate reactions, help with iterative methods, and provide a visual representation of the balancing process, making it easier to understand and apply the techniques.
What does the iterative method (simulation) offer?

+
The iterative method provides a dynamic approach, showing how reactions might occur at a molecular level, which can be invaluable in understanding the underlying mechanisms of complex chemical interactions.