Balancing Combustion Reactions: A Simple Guide
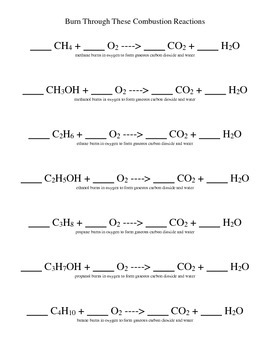
Balancing combustion reactions can seem daunting at first, but with a straightforward approach, it can be much simpler than you think. This guide will walk you through the step-by-step process to balance combustion reactions, ensuring that every chemical equation you write adheres to the law of conservation of mass.
Understanding Combustion Reactions
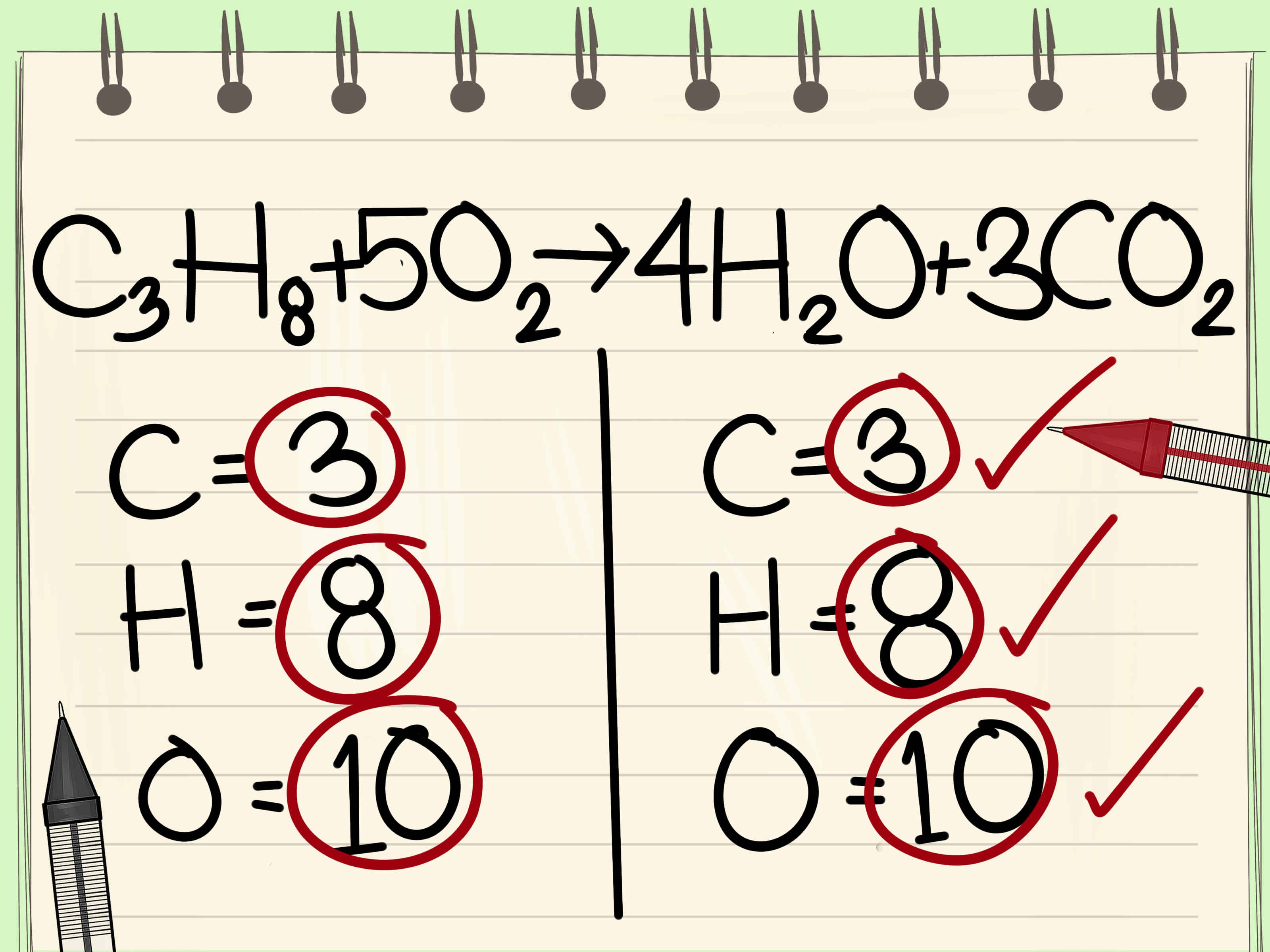
Combustion reactions are redox reactions where substances combine with oxygen to produce energy, usually in the form of heat and light. Here are the key points to understand:
- They involve hydrocarbons or oxygen-containing compounds.
- Most often, the end products are carbon dioxide (CO2) and water (H2O).
- Oxygen gas (O2) is a reactant.

Step-by-Step Guide to Balancing Combustion Reactions

Here’s how to balance a combustion reaction:
- Identify the Reactants and Products: Write down the unbalanced equation with all known reactants and products.
- Balance the Carbon Atoms: Adjust the number of CO2 molecules to match the number of carbon atoms in the hydrocarbon.
- Balance the Hydrogen Atoms: Adjust the number of H2O molecules to match the number of hydrogen atoms.
- Balance the Oxygen Atoms: Add O2 to balance the oxygen atoms on both sides of the equation.
- Final Check: Ensure all atoms are balanced, and coefficients are in their lowest possible integer ratio.
Example of Balancing a Combustion Reaction

Let’s use the combustion of methane as an example:
| Steps | Balanced Equation |
|---|---|
| Unbalanced: | CH4 + O2 → CO2 + H2O |
| Balanced Carbon: | CH4 + O2 → CO2 + H2O |
| Balanced Hydrogen: | CH4 + 2O2 → CO2 + 2H2O |
| Balanced Oxygen: | CH4 + 2O2 → CO2 + 2H2O |

💡 Note: This example illustrates a stoichiometric combustion, meaning there is just enough oxygen to react completely with the fuel.
How to Handle Incomplete Combustion

Incomplete combustion occurs when there isn’t enough oxygen to react with the fuel, leading to the production of carbon monoxide (CO) or elemental carbon (soot):
- Adjust the oxygen to be less than required for complete combustion.
- Include products like CO or soot.
Here’s how incomplete combustion of methane might look:
| Condition | Equation |
|---|---|
| Complete Combustion: | CH4 + 2O2 → CO2 + 2H2O |
| Incomplete Combustion: | CH4 + 1.5O2 → CO + 2H2O |
Checking Your Work

To verify that your combustion reaction is balanced correctly:
- Count the number of atoms of each element on both sides of the equation.
- Ensure the total charge on both sides is balanced if there are ions involved.
- Check the lowest common denominator to simplify coefficients.
Balancing combustion reactions isn't just about following rules; it's about understanding the chemistry behind how fuels burn. Whether you're studying for an exam or working in a lab, these steps will help you tackle combustion equations confidently. Every time you balance a reaction, you're ensuring that the law of conservation of mass holds true, where matter isn't created or destroyed, only transformed.
🔍 Note: Real-world combustion often involves conditions that are not perfectly stoichiometric, leading to variations in the reaction products.
Here are some tips to make balancing combustion reactions even smoother:
- Start with the most complex molecules, often the hydrocarbon fuel.
- Remember that oxygen is typically the element that needs the most adjustment.
- If stuck, try balancing a simpler hydrocarbon first to gain an understanding of the process.
✏️ Note: Practice with different hydrocarbons to build confidence and proficiency in balancing combustion reactions.
What is the difference between complete and incomplete combustion?

+
Complete combustion occurs when there is enough oxygen to react with all the fuel, resulting in CO2 and H2O. Incomplete combustion happens when there’s insufficient oxygen, producing CO or even elemental carbon (soot).
Why is oxygen typically the last element to balance in a combustion equation?

+
Since the number of O2 molecules can be easily adjusted to fit the required oxygen, balancing it last helps to avoid unnecessary revisions.
Can combustion reactions produce energy in the form of light?

+
Yes, combustion reactions often produce light as well as heat. This light emission is what makes flames visible.
How do I simplify the coefficients in the balanced equation?

+
Divide all coefficients by their greatest common divisor. For example, if the coefficients are 2, 4, and 6, divide by 2 to get 1, 2, and 3.