Master Chemical Equation Balancing with Our Answer Key Worksheet

Balancing chemical equations is a fundamental skill every chemistry student must master. Not only does it lay the foundation for understanding stoichiometry, but it also serves as a stepping stone to various chemical calculations and laboratory experiments. This blog post will take you through a detailed exploration of chemical equation balancing, using an answer key worksheet as our primary guide. We will cover the basics, delve into strategies for effective balancing, address common pitfalls, and provide you with the tools to conquer this essential chemical concept.
What is a Chemical Equation?

A chemical equation represents a chemical reaction where reactants, the starting materials, are converted into products. This equation uses chemical symbols to indicate the substances involved:
- Reactants - These are written on the left side of the equation.
- Products - These are placed on the right side.
An arrow (→) points from reactants to products, illustrating the direction of the reaction. Here's an example:
H₂ + O₂ → H₂O
This represents the combustion of hydrogen gas to form water.
The Need for Balancing

Chemical reactions must adhere to the law of conservation of mass, which states that matter can neither be created nor destroyed in a chemical reaction. To satisfy this law:
- Each atom type must be balanced on both sides of the equation.
Basic Principles of Balancing
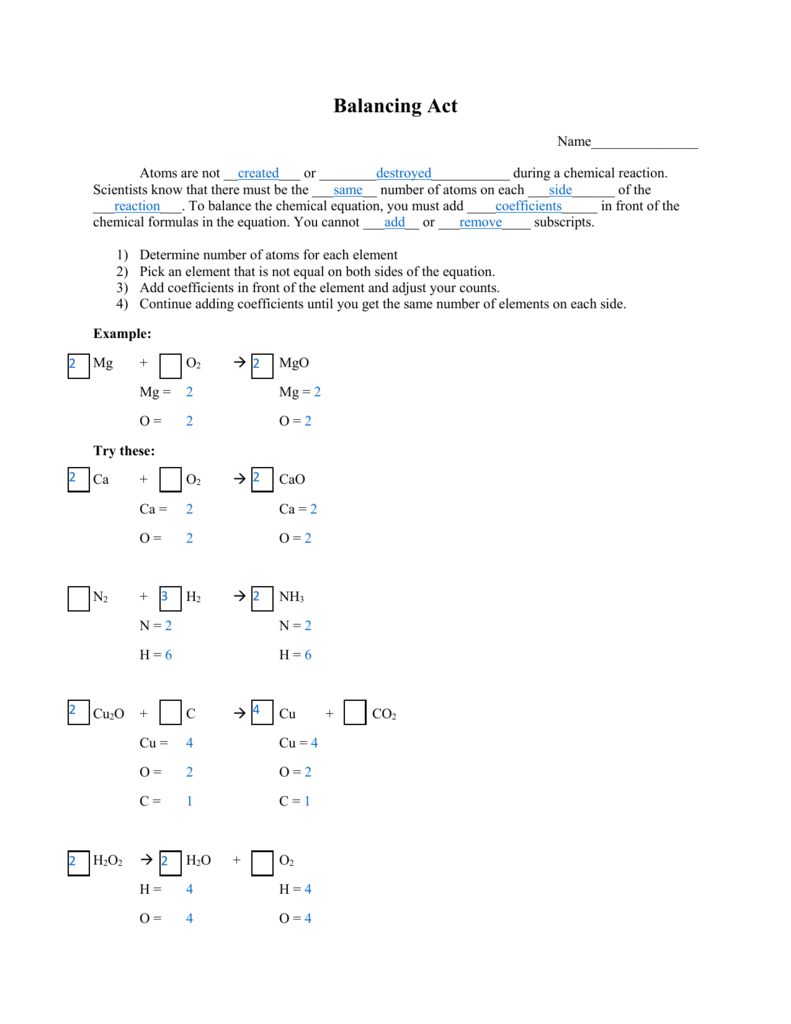
To balance a chemical equation:
- Identify the unbalanced reactants and products.
- Adjust the coefficients (numbers in front of formulas) to balance the atoms.
The following steps are typically followed:
- Start with the most complex molecule containing the most atoms or the most common element.
- Balance hydrogen and oxygen atoms last, as they often appear in many substances.
Strategies for Balancing

Let's consider a worksheet example:
| Equation | Balanced Equation |
|---|---|
| Al + O₂ → Al₂O₃ | 4Al + 3O₂ → 2Al₂O₃ |
| C₂H₆ + O₂ → CO₂ + H₂O | 2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O |

Step-by-Step Example:

- Start with Al + O₂ → Al₂O₃:
- Balance Al atoms: 2Al + O₂ → Al₂O₃ → 4Al + 3O₂ → 2Al₂O₃
- Balance O atoms: Now balanced with the adjusted coefficients.
- For C₂H₆ + O₂ → CO₂ + H₂O:
- Balance C atoms: 2C₂H₆ + O₂ → 4CO₂ + H₂O
- Balance H atoms: 2C₂H₆ + O₂ → 4CO₂ + 6H₂O
- Balance O atoms: 2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O
Common Pitfalls and Solutions

Balancing can be tricky due to several common issues:
- Incorrect coefficient placement: Always adjust coefficients, not subscripts.
- Forgetting polyatomic ions: Balance polyatomic ions as units if they remain intact.
- Overlooking fractions: If you get a fraction coefficient, multiply all coefficients by the denominator to clear it.
Advanced Balancing Techniques

Some equations require additional strategies:
- Inspection Method: Simple equations balanced by inspection.
- Linear Equation Approach: For complex reactions, solve as a system of linear equations.
- Oxidation-Reduction Balancing: Redox reactions can require special attention to changes in oxidation numbers.
Balancing Complex Equations

Here’s an advanced example to illustrate:
KMnO₄ + HCl → KCl + MnCl₂ + Cl₂ + H₂O
Balanced:
2KMnO₄ + 16HCl → 2KCl + 2MnCl₂ + 5Cl₂ + 8H₂O
Practical Application:

In laboratory experiments, balanced equations are crucial for:
- Calculating the stoichiometric amounts of reagents.
- Predicting the amount of product formed.
- Understanding reaction pathways and kinetics.
⚠️ Note: When balancing equations in a lab setting, precision in measurements and adherence to safety protocols are imperative.
Having navigated through the art of balancing chemical equations, we've explored why balancing is essential, how to balance simple to complex equations, and the strategies to avoid common errors. Mastering this skill not only enhances your understanding of chemistry but also prepares you for more advanced chemical concepts. With practice using worksheets like our answer key, students can achieve proficiency in balancing equations, making complex reactions more approachable.
Why must chemical equations be balanced?

+
Chemical equations must be balanced to adhere to the law of conservation of mass, ensuring that the number of atoms of each element is the same on both sides of the equation.
How can I check if my chemical equation is balanced?

+
Count the atoms of each element on both sides of the equation. If the totals are equal, the equation is balanced.
What do I do if my equation has a fractional coefficient?

+
Multiply all coefficients by the denominator to clear the fraction and balance the equation with whole numbers.