5 Essential Tips for Chemical Equation Balancing

Balancing chemical equations is a crucial skill for students and professionals in chemistry, helping to understand the stoichiometry of reactions and ensuring conservation of mass in chemical processes. While it might seem daunting at first, mastering this skill simplifies future chemical equations and reactions significantly. Here are 5 essential tips to streamline the process of balancing chemical equations:
Practical Strategies for Balancing Chemical Equations

Balancing a chemical equation involves adjusting the coefficients of reactants and products to ensure that the number of atoms of each element is the same on both sides of the equation. Here are some practical strategies to help you achieve this:
- Conservation of Mass: The total mass of the reactants must equal the total mass of the products. This is a fundamental law in chemistry.
- Check Each Element: Balance the equation by addressing one element at a time, typically starting with the most complex molecule or the one with the highest count of atoms.
- Use Fractions: In the initial stages, you might use fractions to balance equations. Later, these can be converted to whole numbers by multiplying all coefficients by the lowest common denominator.
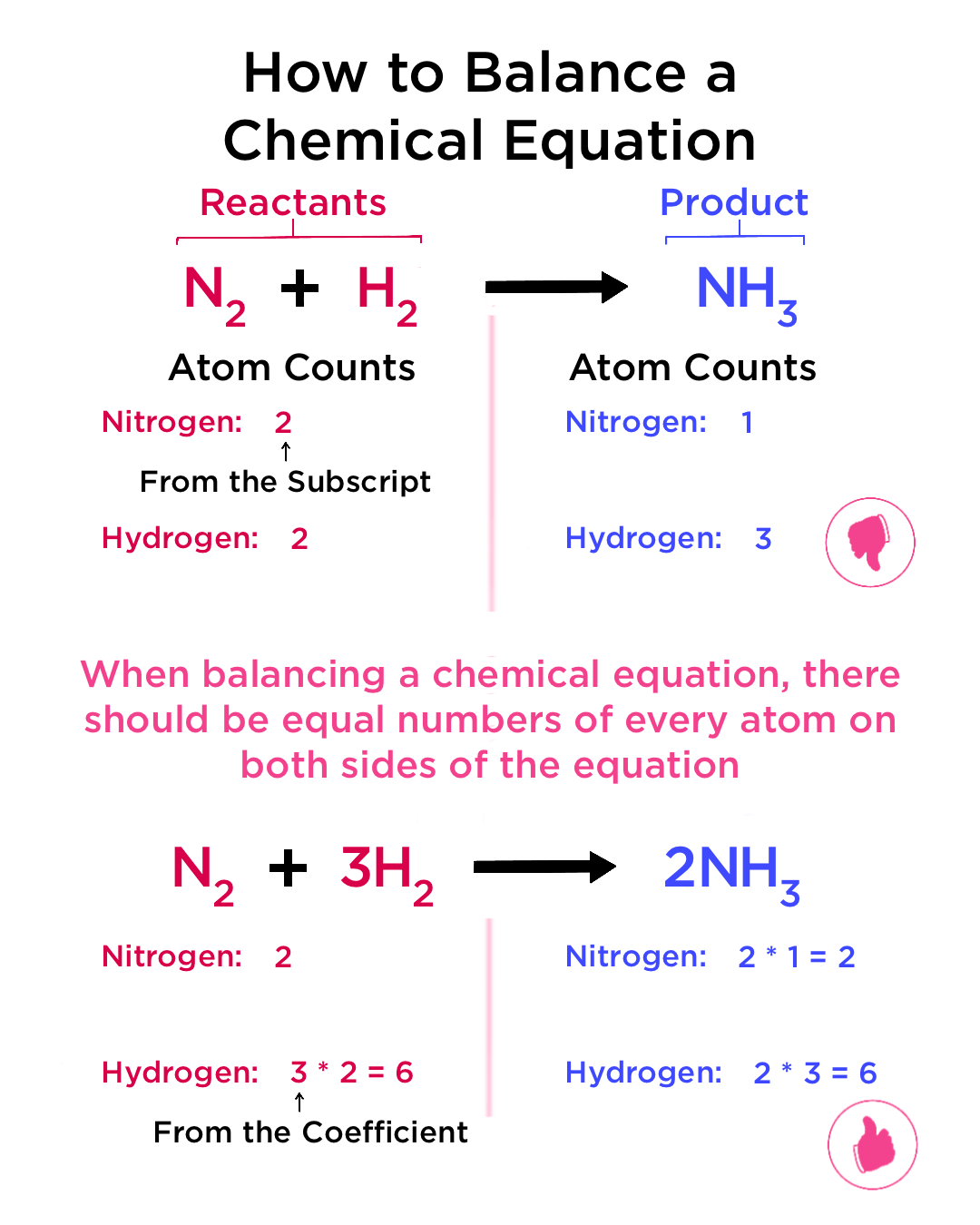
Start with a Template Equation

To make the process of balancing less overwhelming, start with a template of the reaction. Here’s how you might approach it:
- Write down the unbalanced chemical equation.
- Count the number of atoms of each element on both sides.
- Choose an element to balance first, often the one with the most atoms or in a complex molecule.
- Adjust coefficients to balance that element, then move to the next.
Let’s illustrate this with an example:

🧪 Note: Balancing can often seem complex with polyatomic ions; in such cases, balance these ions as a unit if they appear unchanged in both reactants and products.
Balancing using Redox Reactions
Redox (Reduction-Oxidation) reactions require a special approach due to the transfer of electrons. Here's how to balance such equations:
- Identify the Oxidation States: Assign oxidation numbers to all elements in the reaction.
- Write the Half-Reactions: Separate the equation into oxidation and reduction half-reactions.
- Balance the Half-Reactions: Balance both mass and charge for each half-reaction.
- Combine the Half-Reactions: Ensure the number of electrons lost equals the number gained by making electron counts match.
- Add Water and Hydrogen Ions: For aqueous solutions, balance the equation using H2O and H+ if needed.
| Step | Description |
|---|---|
| 1 | Assign Oxidation Numbers |
| 2 | Write Half-Reactions |
| 3 | Balance Atoms and Charges |
| 4 | Combine Half-Reactions |
| 5 | Add H2O and H+ |

Check and Adjust the Equation

After balancing, always check your work to ensure:
- The number of atoms of each element is the same on both sides.
- Electrical charges are balanced if the reaction is in aqueous solution or involves ions.
- The equation doesn't contain fractions or negative coefficients (convert them to whole positive numbers).
⚗️ Note: If you find yourself stuck, try re-evaluating your approach; sometimes a different element or ion might be the key to balancing the entire equation.
Practice with Common Reactions

Chemical equation balancing is a skill that requires practice. Familiarize yourself with some common reactions to enhance your balancing proficiency:
- Combustion reactions
- Neutralization reactions
- Decomposition reactions
The more you practice, the more you'll recognize patterns and develop an intuitive sense for how to balance equations quickly and accurately.
Utilize Modern Balancing Tools

In an age of digital aids, balancing chemical equations can be made easier with:
- Online equation balancers
- Chemical equation apps
- Excel spreadsheets programmed for balancing
These tools can help verify your work or guide you through the process. However, manual balancing is still essential to understanding the underlying principles of chemistry.
By implementing these strategies and tips, you'll find that balancing chemical equations becomes less of a chore and more of a logical exercise. Remember, the key is in understanding the fundamentals of chemical reactions, practicing regularly, and not being afraid to rethink your approach if something isn't working.
The journey to becoming proficient at balancing equations will enhance your chemical education and deepen your appreciation for the precision and balance inherent in chemical reactions.
Why is it important to balance chemical equations?

+
Balancing chemical equations ensures that the law of conservation of mass is obeyed, which states that matter cannot be created or destroyed. This process also helps in understanding the stoichiometry and predicting reaction outcomes.
What is the difference between oxidation and reduction?

+
Oxidation is the loss of electrons by a substance, increasing its oxidation number, while reduction is the gain of electrons, decreasing the oxidation number. These two processes occur simultaneously in a redox reaction.
Can chemical equations always be balanced with whole numbers?

+
Most often, yes. However, if fractions are initially necessary, they can be converted to whole numbers by multiplying all coefficients by the lowest common denominator to maintain the ratio of reactants and products.
How can one check if an equation is balanced?

+
By counting the number of atoms of each element on both sides of the equation and ensuring they are equal. Additionally, for redox reactions, ensure the charge balance is maintained.