Balancing Act Worksheet: Your Answer Key Guide

The Balancing Act Worksheet is an essential tool used to enhance students' understanding of chemistry concepts, specifically in the area of chemical equations and stoichiometry. Whether you are a high school student struggling with balancing chemical equations or an educator looking to refine teaching methods, this worksheet and its answer key serve as crucial learning aids. This guide aims to walk you through how to use the Balancing Act Worksheet effectively and provide insights into various techniques and tips for mastering this fundamental aspect of chemistry.
Understanding the Importance of Balancing Chemical Equations


Chemical reactions are inherently about balancing. Balancing equations ensures conservation of mass, one of the fundamental laws of chemistry. Here’s why it matters:
- Stoichiometry and Predictions: Knowing how much reactant is needed or how much product can be produced.
- Reaction Mechanisms: Balancing can help predict how a reaction proceeds.
- Thermodynamics: Understanding the energy changes within a balanced system.
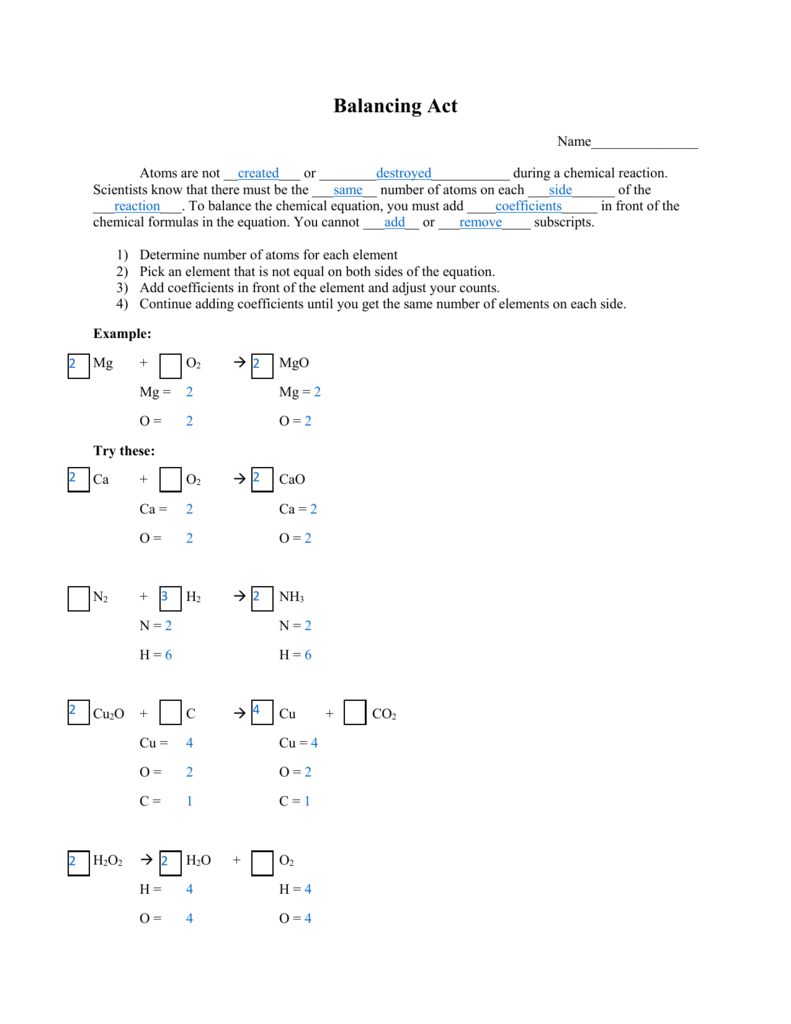
How to Use the Balancing Act Worksheet

The Balancing Act Worksheet is typically divided into three primary sections:
1. The Chemical Equation

This section includes an unbalanced chemical equation. Here are the steps to balance it:
- Identify reactants and products: Note down the elements present on both sides.
- Count atoms: Calculate the number of each atom on both sides.
- Adjust coefficients: Begin adjusting the smallest numbers to balance the equation, ensuring you don’t change subscripts.
2. Balancing Techniques

Balancing can be a puzzle. Here are a few techniques:
- Inspection Method: Simple trial and error.
- Systematic Approach: Balancing by elements, ensuring you balance the most complex molecule first.
- Least Common Multiple: Using LCM to balance complex reactions.
3. Applying the Answer Key

After attempting to balance the equations on your own, the answer key provides:
- Confirmation: Check if your balanced equation matches the provided solution.
- Learning Points: Understand where you might have gone wrong or learn new methods to balance the equation.
📝 Note: The answer key is not just a tool for checking answers but also a rich learning resource to understand the logic behind balancing equations.
Advanced Strategies for Balancing Equations

For more complex reactions, here are some advanced strategies:
- Use of Oxidation States: Understanding oxidation-reduction reactions can simplify balancing.
- Grouping Coefficients: When you can’t balance individual elements, balance groups or molecular units.
- Linear Algebra Approach: For extremely complex equations, employ mathematical techniques to find solutions.
Tips for Teaching Balancing Equations
Here are practical tips for educators:
- Start with Simple Reactions: Begin with single replacement or decomposition reactions.
- Practice, Practice, Practice: Use interactive tools, puzzles, or gamification to engage students.
- Relate to Real Life: Discuss chemical reactions happening around us, making the concept more tangible.
Key Takeaways for Students

The process of balancing chemical equations not only prepares you for higher-level chemistry but also:
- Enhances Problem Solving Skills: It’s an excellent exercise in logical thinking and mathematical precision.
- Prepares for Future Chemistry Topics: Balancing equations is foundational for understanding reaction kinetics, thermodynamics, and even organic synthesis.
- Fosters Precision: It requires meticulous attention to detail, a skill invaluable in science.
The Balancing Act Worksheet along with its answer key is more than just a practice sheet; it's a learning journey. By mastering the art of balancing equations, you not only learn chemistry but also cultivate a scientific approach to problem-solving. The key to success lies in understanding the principles, practicing diligently, and embracing the logic behind each chemical reaction.
Why is it important to balance chemical equations?

+
Balancing chemical equations ensures that the law of conservation of mass is followed, meaning the number of atoms of each element remains constant during the reaction. This balancing provides a foundation for stoichiometry, allowing scientists to predict the amount of products formed or reactants consumed, which is crucial for chemical synthesis and analysis.
What are some common mistakes students make when balancing equations?

+
Common mistakes include changing the subscripts within the compounds instead of using coefficients to balance atoms, not considering polyatomic ions as units, forgetting to balance hydrogen and oxygen last, and trying to balance equations through trial and error without systematic approach.
How can teachers make balancing chemical equations more engaging?

+
Teachers can engage students by introducing hands-on activities, using educational software or apps for virtual balancing, organizing competitions or games related to chemical reactions, and connecting the balancing act to real-world examples like cooking or chemical engineering processes.