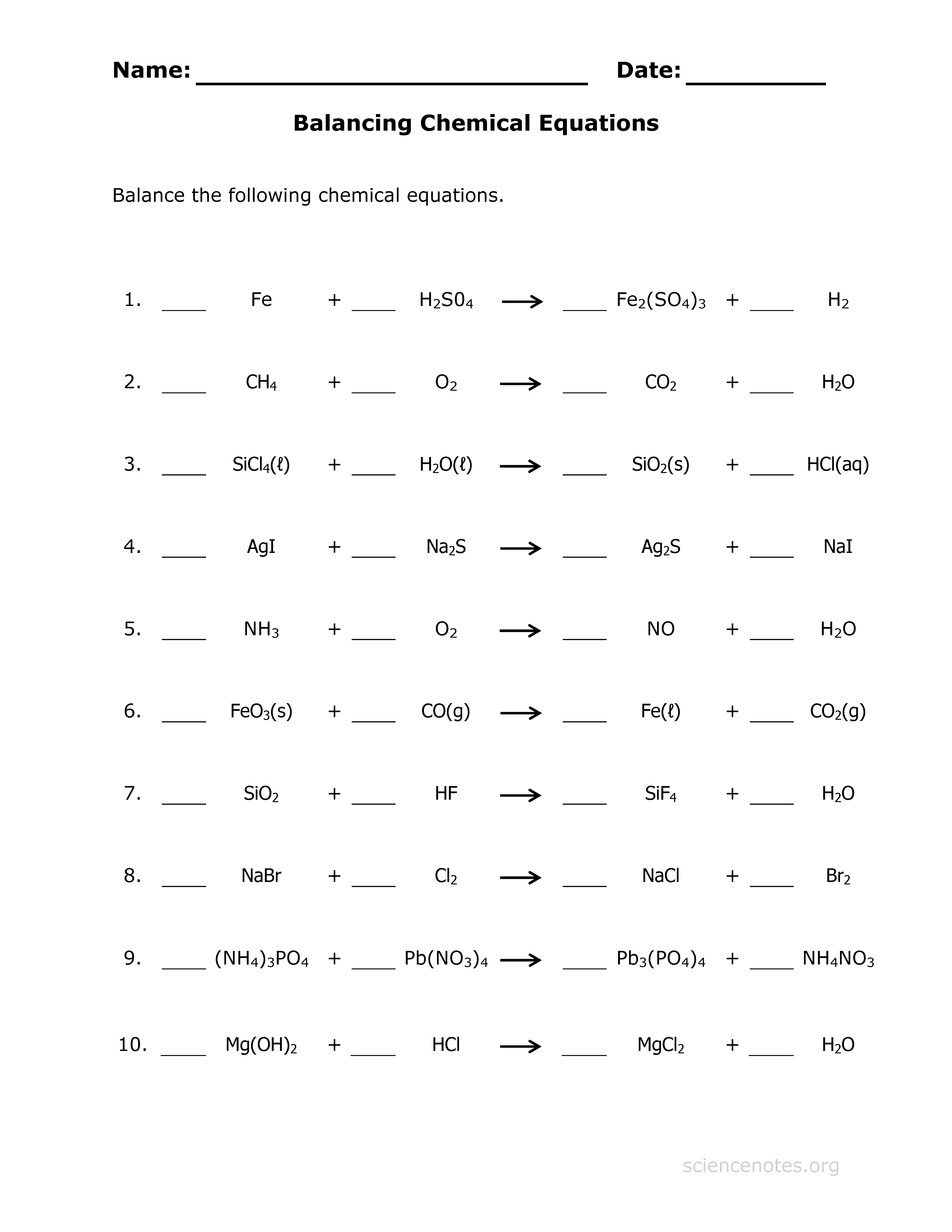
Master Balancing Chemical Reactions with Our Free Worksheet

Balancing chemical equations is a fundamental skill in chemistry, crucial for understanding chemical reactions and stoichiometry. Whether you're a high school student or a university undergrad, mastering this skill can make or break your grasp on chemistry. But fear not! With the right resources, anyone can become adept at balancing chemical reactions. Today, we delve into using our specially designed free balancing chemical reactions worksheet to enhance your understanding and ability.
Why Balance Chemical Reactions?
Balancing a chemical equation is akin to ensuring the law of conservation of mass is respected in a chemical reaction. Here are some reasons why balancing is essential:
- Mass Conservation: Atoms are neither created nor destroyed in a reaction. They are merely rearranged.
- Reaction Ratios: It shows the relative amounts of reactants and products, which is vital for calculations in experiments.
- Understanding Stoichiometry: It lays the groundwork for quantitative analysis in chemical reactions.
Steps to Balance Chemical Equations

Balancing chemical reactions can seem daunting, but here’s a straightforward approach:
- Identify Reactants and Products: Begin by recognizing all the atoms on both sides of the equation.
- Count Atoms: Tally up the number of each type of atom on both sides.
- Adjust Coefficients: Change the coefficients of the formulas to balance each atom. Never alter subscripts within a compound.
- Double-check: Ensure that all atoms are now balanced. If not, reevaluate your coefficients.
📝 Note: Always balance elements other than hydrogen and oxygen first, as these often appear in water or acids and can be adjusted last.
Utilizing Our Free Worksheet

Our balancing chemical reactions worksheet is crafted to guide you through the process, offering step-by-step instructions along with practice problems. Here’s how to get the most out of it:
- Worksheet Structure: It’s divided into sections with instructions, examples, and problems.
- Practice: Start with simpler equations and progress to more complex reactions.
- Check Your Work: Solutions are provided at the end of each section for immediate feedback.
Common Pitfalls in Balancing Equations

Even seasoned chemists can trip up. Here are common mistakes to avoid:
- Ignoring Phases: The state of matter (solid, liquid, gas, aqueous) is often forgotten, but it matters in some reactions.
- Changing Subscripts: Balancing should only affect coefficients, not the subscripts of elements within compounds.
- Overlooking Polyatomic Ions: Remember, polyatomic ions often remain intact in reactions.
Advanced Balancing Techniques

For more complex reactions, consider these advanced methods:
| Method | Description | Example |
|---|---|---|
| Algebraic Method | Assign variables to coefficients and set up a system of equations based on atom counts. | Balancing combustion reactions. |
| Fractional Balancing | Use fractions to balance equations initially, then multiply through to clear fractions. | Redox reactions with multiple species. |
| Half-Equation Method | Balance redox reactions by separating oxidation and reduction half-reactions. | Oxidation-reduction reactions. |

To wrap up, mastering the art of balancing chemical reactions not only boosts your understanding of chemical processes but also your confidence in tackling chemistry problems. Our free worksheet is an excellent tool for practice, offering a range of exercises from the basics to more advanced balancing techniques. By following the guidelines provided, you can transform this often intimidating task into a manageable and even enjoyable part of your learning journey.
How do I know if a reaction is balanced?

+
A chemical equation is balanced when the number of each type of atom on the reactants’ side equals the number on the products’ side.
Can I balance equations by changing subscripts?

+
No, you should never change the subscripts within a chemical formula. Instead, adjust the coefficients in front of compounds.
What are polyatomic ions?

+
Polyatomic ions are groups of atoms that carry a charge and behave as single units in chemical reactions.