5 Tips to Easily Balance Chemical Equations
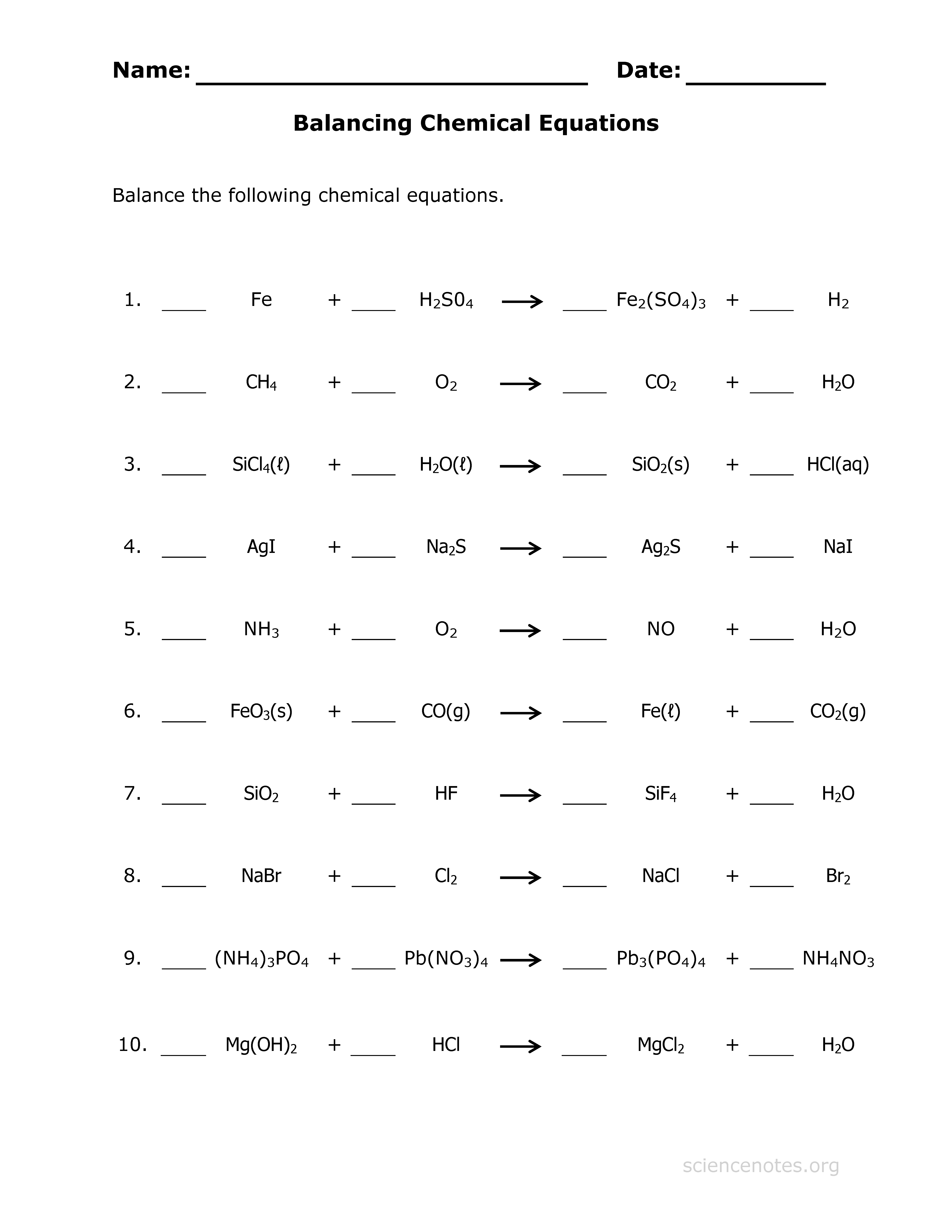
In the world of chemistry, balancing chemical equations is a fundamental skill that forms the backbone of understanding chemical reactions. Whether you're a student, a teacher, or just a curious individual, mastering the art of balancing equations can demystify chemical processes and help in solving complex problems. Here are five essential tips to help you balance chemical equations with ease and accuracy.
1. Understand the Basics
Why do we balance equations? Chemical equations need to be balanced to satisfy the Law of Conservation of Mass, which states that matter cannot be created or destroyed in chemical reactions. This means that the number of atoms of each element must be the same on both sides of the equation.
Here are the steps to get started:
- Identify all the elements in the equation.
- Count the number of atoms for each element on both sides.
- Adjust coefficients to balance the number of atoms.
🔍 Note: Balancing equations doesn’t involve altering subscripts; you only adjust coefficients.
2. Use the Inspection Method


The inspection method, or hit-and-trial method, is one of the simplest ways to balance equations:
- Start with the most complex molecule or the one with the highest number of atoms.
- Balance atoms that appear in only one reactant and one product first.
- Work your way to the others, ensuring you keep the overall count consistent.
✅ Note: This method might not work for all equations, especially if they involve complex polyatomic ions or high oxidation states.
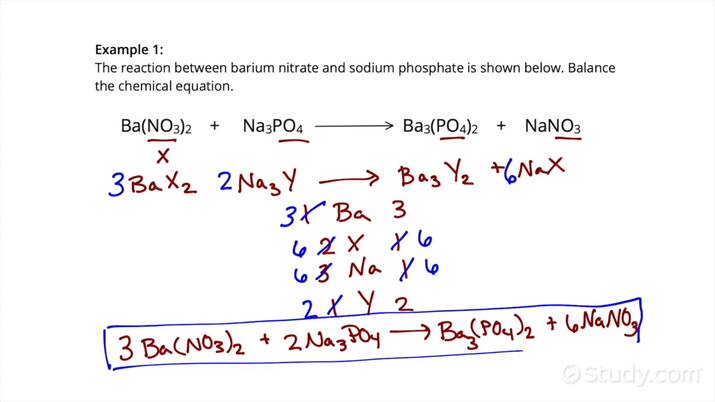
3. Leverage The Algebraic Method

For more complex reactions, the algebraic approach can be very useful:
- Assign variables (like ( x, y, z )) to coefficients.
- Set up equations based on the number of atoms for each element.
- Solve these equations simultaneously to find the values of the variables.
This method ensures accuracy but can be time-consuming. Here’s a simple example:
| Balancing Equation | Step | Result |
|---|---|---|
| [ \text{C}_x \text{H}_4 + \text{O}_y \rightarrow \text{CO}_2 + \text{H}_2\text{O} ] | Set x = 2 (for the simplest hydrocarbon) | [ \text{C}_2\text{H}_4 + \text{O}_y \rightarrow 2\text{CO}_2 + 2\text{H}_2\text{O} ] |
| Solve for oxygen: | ( 3 + y = 6 + 2 \Rightarrow y = 5 ) |

📝 Note: This method requires knowledge of algebra, making it beneficial for advanced learners.
4. Grouping and Polyatomic Ions

If your equation involves complex ions:
- Balance polyatomic ions as a whole if they appear unchanged on both sides.
- Look for the grouping of atoms to simplify the balancing process.
For instance, in the equation:
[ \text{Pb(NO}_3\text{)}_2 + \text{K}_2\text{S} \rightarrow \text{PbS} + \text{KNO}_3 ]
You balance ( \text{NO}_3^- ) as a single unit.
🔗 Note: If the ion changes form, like in a redox reaction, consider the individual atoms within the ion.
5. Practice, Practice, Practice

The more you balance, the better you get:
- Use online tools for practice.
- Join study groups or forums where equations are discussed.
- Try balancing different types of reactions to expand your skills.
🧑🎓 Note: Repetition not only hones your balancing skills but also helps in understanding various chemical reactions.
To wrap up, balancing chemical equations is not just about numbers; it's about understanding the nature of reactions, stoichiometry, and the underlying chemical principles. By following these tips, you'll not only improve your ability to balance equations but also deepen your appreciation for chemistry. Remember to practice regularly, employ various methods, and keep in mind the conservation laws that dictate the balance. Through consistent effort, you'll find that what once seemed daunting becomes a straightforward and rewarding task.
What does balancing an equation signify in terms of chemical reactions?

+
Balancing an equation shows that mass is conserved in chemical reactions, meaning that the total mass of reactants equals the total mass of products. This represents the Law of Conservation of Mass.
Can the algebraic method be used for all chemical equations?

+
While the algebraic method can solve all balanced equations, it might be overkill for simple reactions where the inspection method suffices. However, for complex equations, algebraic approaches ensure accuracy and efficiency.
Is there a limit to how complex the chemical equations can get for balancing?

+
In theory, there’s no limit to how complex an equation can be, but practical applications often require simplifying large equations into smaller, manageable parts or using computer programs for accuracy in balancing.