Atomic Structure Worksheet Key: Master Chemistry Easily

In the vast expanse of the chemical universe, understanding atomic structure is fundamental. Whether you're a high school student tackling your first chemistry lesson or an enthusiast exploring deeper into the mysteries of matter, grasping the concept of atomic structure is crucial. In this detailed exploration, we'll dive into an atomic structure worksheet key, designed to simplify this essential concept, helping you master chemistry with ease.
What is Atomic Structure?

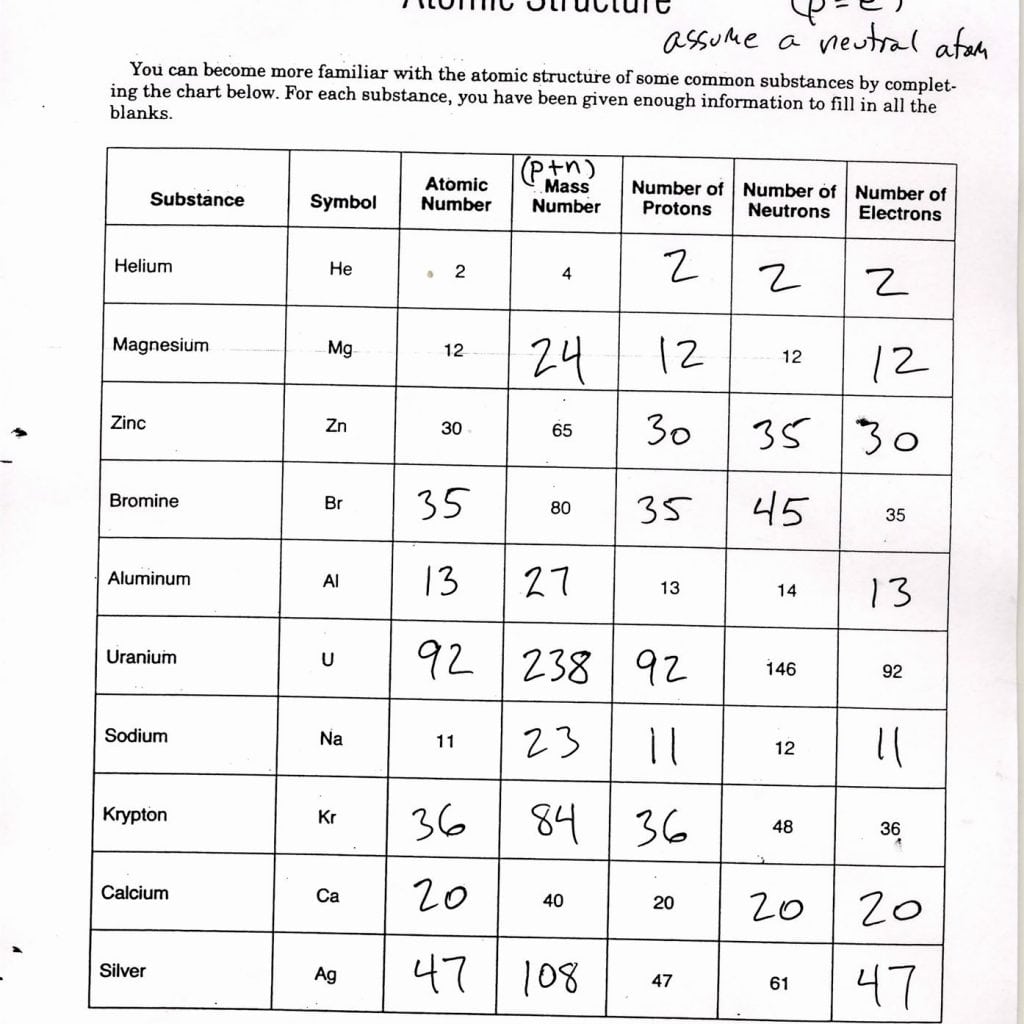
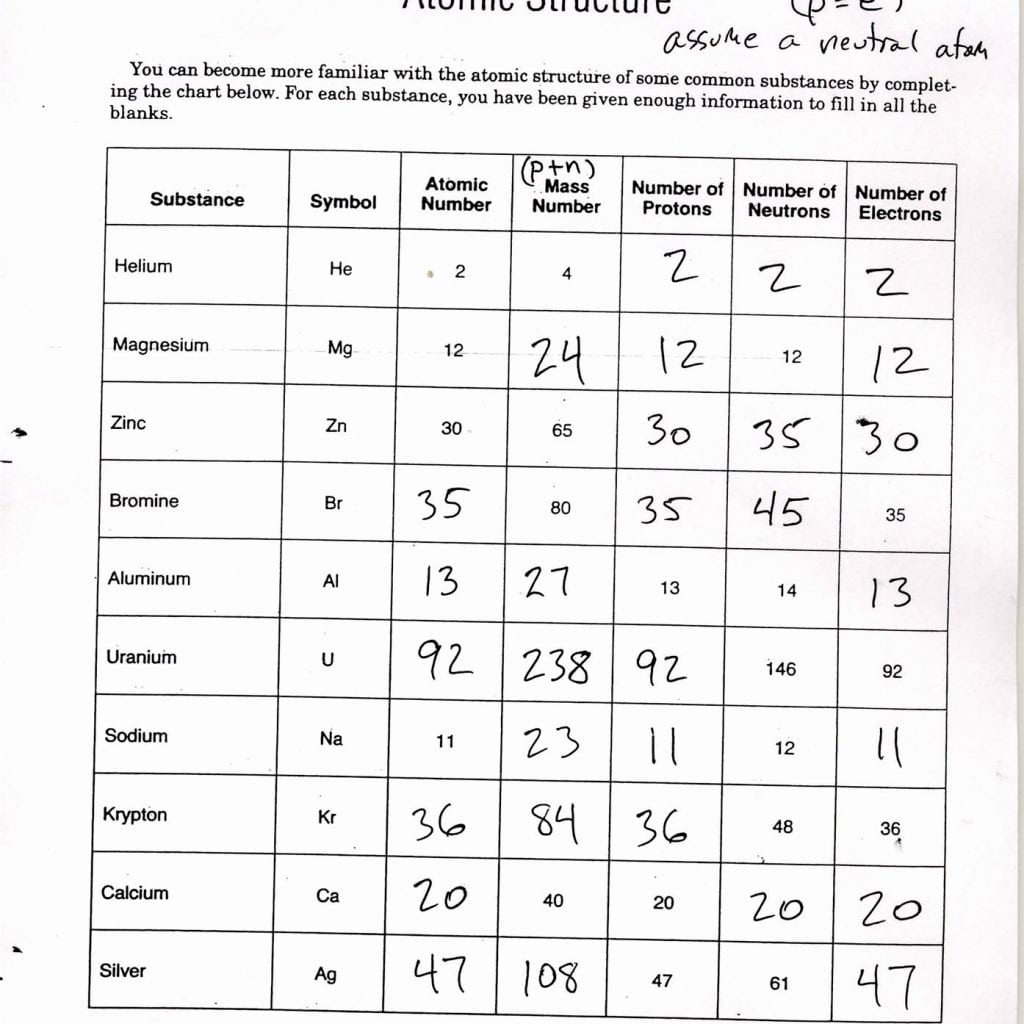
Atomic structure refers to the composition and arrangement of subatomic particles within an atom. These particles include:
- Protons: Positively charged particles located in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus.
- Neutrons: Neutral particles also located in the nucleus alongside protons.
The Building Blocks of Matter
Atoms, the smallest unit of matter, are composed of these subatomic particles:
- Protons give the element its identity or atomic number. For example, hydrogen has one proton, thus its atomic number is 1.
- Neutrons contribute to the mass of the atom, and their number can vary to form isotopes.
- Electrons determine the atom’s chemical behavior, as they participate in bonding.
Atomic Mass and Isotopes

Here’s where things get interesting with atomic mass:
- The atomic mass of an element is an average mass of all its isotopes, weighted by their natural abundance.
- Isotopes are variants of an element with different numbers of neutrons but the same number of protons. For instance, carbon-12, carbon-13, and carbon-14 are all isotopes of carbon, with 6, 7, and 8 neutrons respectively.
The Electron Configuration

How electrons arrange themselves around the nucleus is key to an element’s chemical properties:
- Electrons occupy energy levels or shells, each of which can hold a maximum number of electrons.
- The valence electrons, those in the outermost shell, play the critical role in chemical bonding.
Let's look at an example using hydrogen:
- Hydrogen has one electron in its first shell. The configuration is written as 1s1.
Building the Atomic Structure
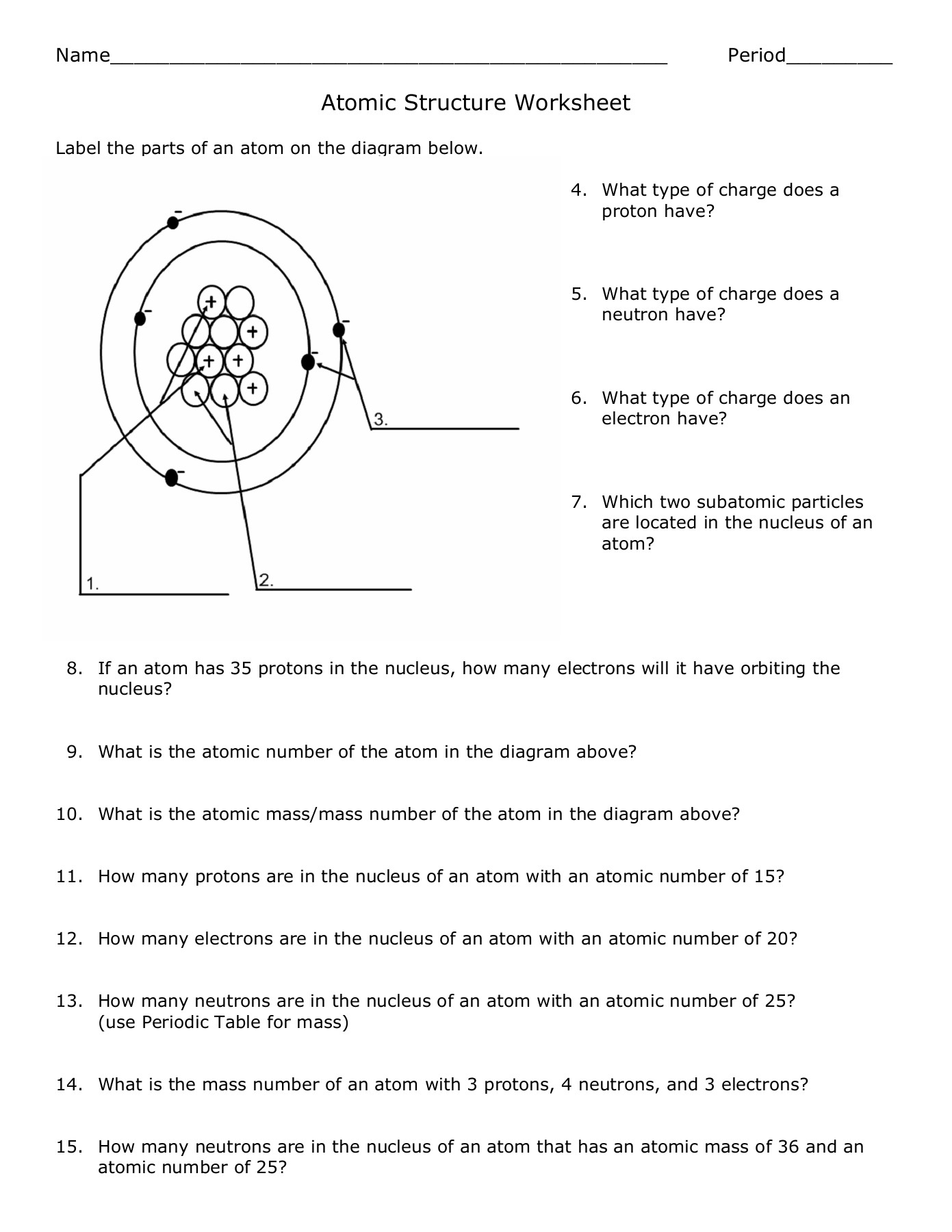
Here is a step-by-step guide to constructing the atomic structure:
- Determine the Atomic Number: This tells you the number of protons.
- Calculate the Mass Number: To find the mass number, sum the protons and neutrons.
- Arrange Electrons: Follow the Aufbau Principle, where electrons fill the lowest energy orbitals first.
- Identify Valence Shell: Know which shell contains the valence electrons.
- Note Isotopes: Remember isotopes have different numbers of neutrons.
⚗️ Note: The number of neutrons can be different in isotopes, but the proton count remains the same.
Practical Applications of Atomic Structure

Understanding atomic structure isn’t just theoretical; it has real-world applications:
- Medical Imaging: Radioisotopes, which are atoms with unstable nuclei that emit radiation, are used in diagnostics and treatments.
- Nuclear Energy: The splitting (fission) or fusing (fusion) of atomic nuclei releases enormous amounts of energy.
- Chemistry and Industry: Knowing the electron structure is vital for predicting how substances will react or bond.
Wrapping Up

In mastering chemistry, understanding the atomic structure is a foundational step. From protons, neutrons, and electrons to isotopes and electron configurations, each component contributes uniquely to the behavior and properties of elements. This worksheet key not only simplifies these complex ideas but also connects theory with practical applications, enhancing your journey into the world of chemistry.
Why is the atomic number important?

+
The atomic number determines the identity of the element, as it indicates the number of protons in the nucleus.
What are isotopes and why do they matter?
+
Isotopes are atoms of the same element with different numbers of neutrons. They are important because they can have different physical properties and are used in various applications, from medicine to power generation.
Can you change an atom’s atomic structure?

+
Yes, through nuclear reactions, you can alter the number of protons (transmutation) or neutrons (isotope creation).
How do electrons affect chemical behavior?

+
Electrons in the outermost shell, known as valence electrons, are involved in bonding and reactions, determining the chemical properties of the element.