5 Must-Know Answers for Atomic Structure Worksheet
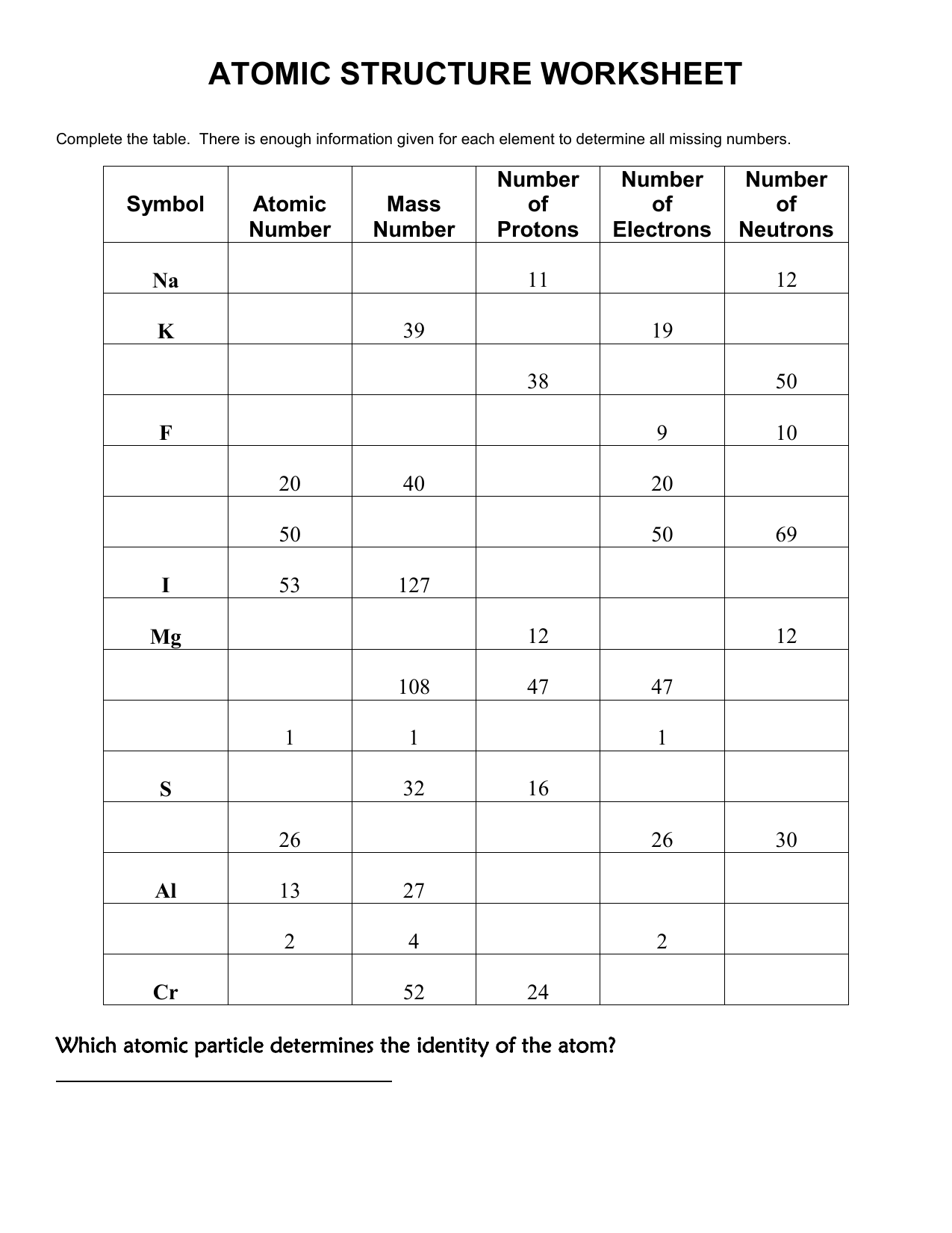
Chemistry is a fascinating field that unveils the intricacies of the world at its molecular and atomic levels. One of the fundamental concepts in chemistry is atomic structure, which explains how atoms are made up and how they interact to form different substances. For many students, understanding atomic structure can be challenging but is crucial for mastering higher-level chemistry topics. This blog post will delve into the essentials of atomic structure, providing key answers and insights to help you navigate the complexities of chemistry worksheets on this subject.
Understanding Atoms

An atom is the basic unit of matter and comprises three main particles: protons, neutrons, and electrons:
- Protons: Positively charged particles found in the nucleus of an atom. Each proton has a charge of +1.
- Neutrons: Uncharged particles in the nucleus; they have nearly the same mass as protons.
- Electrons: Negatively charged particles that orbit the nucleus in specific energy levels or shells.
The Nucleus and Electron Shells


At the heart of every atom is the nucleus, which contains protons and neutrons. The atomic number of an element is defined by the number of protons in its nucleus. For example, oxygen has an atomic number of 8, indicating 8 protons. Here’s how electron shells work:
- The first shell can hold up to 2 electrons.
- From the second shell onward, each can hold up to 8 electrons, following the octet rule.
- Electrons fill the shells in order of increasing energy, with lower energy shells filled first.
Isotopes: Variants of an Element

Isotopes are atoms of the same element with a different number of neutrons, thus having the same atomic number but a different mass number. Here are some key points about isotopes:
- Isotopes have nearly identical chemical behavior since the number of electrons doesn’t change.
- The mass number, which is the sum of protons and neutrons, varies between isotopes.
- Isotopes can be stable or radioactive, with the latter eventually decaying to other elements.
Atomic Mass and Mass Number
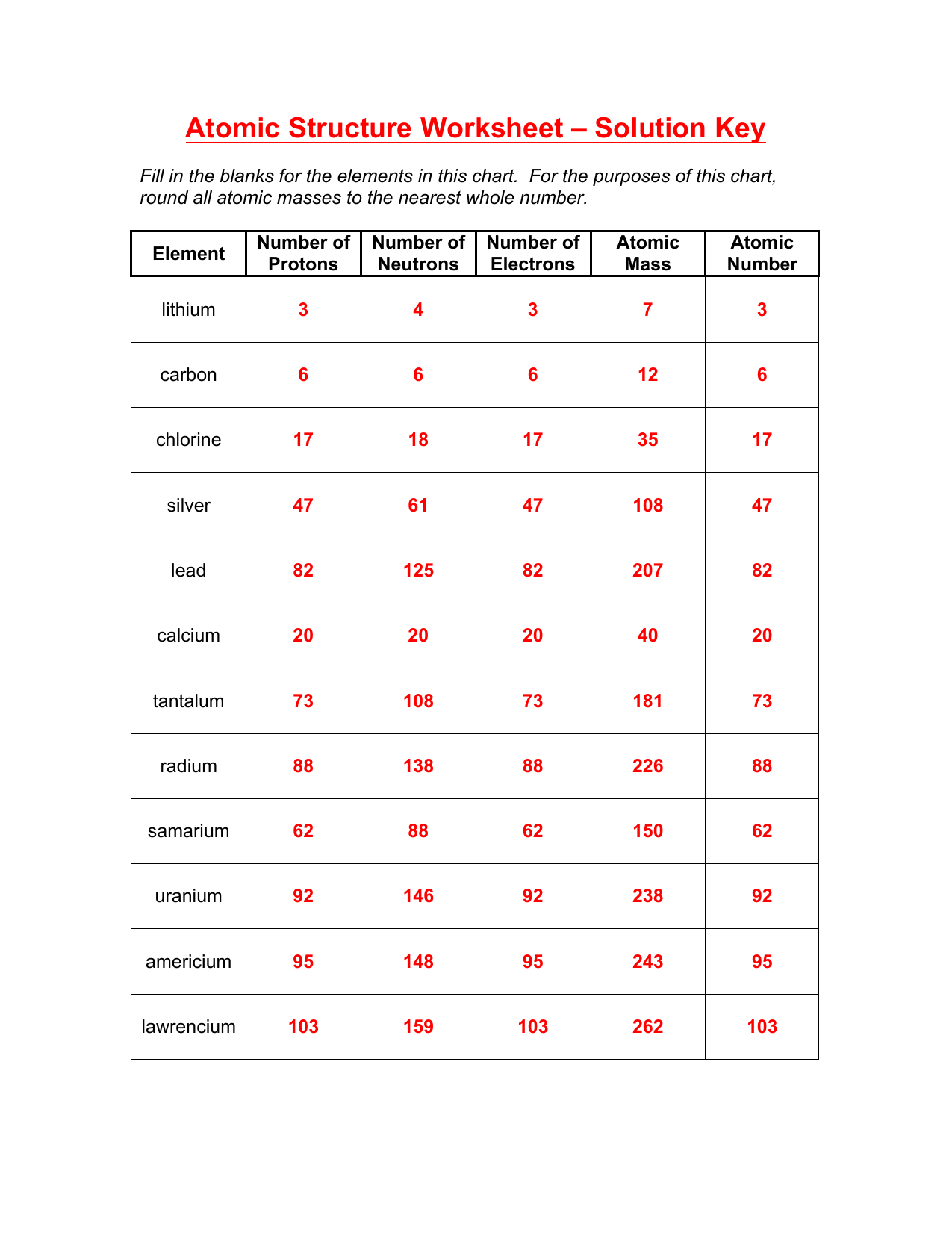
The mass number is the total number of protons and neutrons in an atom’s nucleus:
- To find the mass number, simply add the number of protons to the number of neutrons.
- The atomic mass, or relative atomic mass, is a weighted average of the mass numbers of all naturally occurring isotopes, accounting for their relative abundance.
| Element | Symbol | Atomic Number | Mass Number | Atomic Mass | Number of Neutrons |
|---|---|---|---|---|---|
| Carbon-12 | C | 6 | 12 | 12.011 | 6 |
| Carbon-14 | C | 6 | 14 | 12.011 | 8 |

📚 Note: When calculating atomic mass, consider the abundance of each isotope in nature. The atomic mass might not be a whole number due to the natural isotopic distribution.
Electron Configuration

Electron configuration describes how electrons are distributed among the energy levels or shells of an atom. Here’s the process:
- Start filling electrons in the lowest energy level (1s) first.
- Follow the Aufbau Principle, Pauli Exclusion Principle, and Hund’s Rule for placement.
Importance of Atomic Structure

Understanding atomic structure is vital for several reasons:
- It explains the behavior of elements in chemical reactions.
- It allows prediction of bond formation and properties of compounds.
- It underpins the study of nuclear reactions and radioactivity.
The exploration of atomic structure helps demystify the chemical world, enabling us to understand and manipulate the properties of substances for various applications, from medicine to energy production.
What is the difference between an atom and an ion?

+
An atom has an equal number of protons and electrons, making it electrically neutral. An ion, however, is an atom or molecule that has either gained or lost electrons, thus acquiring a net positive or negative charge. For instance, a sodium atom (Na) can lose an electron to become a Na+ ion, which is a cation. Conversely, a chlorine atom (Cl) can gain an electron to form a Cl- ion, an anion.
Why are isotopes important in atomic structure?

+
Isotopes are crucial for several reasons:
- Chemical Behavior: Isotopes of an element have similar chemical properties due to the same number of electrons, but they can behave differently in reactions or biological systems due to slight mass differences.
- Nuclear Applications: Some isotopes are radioactive, which is vital for medical imaging, dating historical artifacts, and powering nuclear reactors.
- Environmental Studies: Isotope ratios can trace pathways of substances in ecosystems, helping to understand nutrient cycling or the impact of human activities on the environment.
How do electrons fill into the electron shells?

+
Electrons fill into electron shells according to these rules:
- Aufbau Principle: Electrons occupy the lowest energy level first.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, with opposite spins.
- Hund’s Rule: For degenerate orbitals (same energy level), electrons occupy them singly before pairing up.