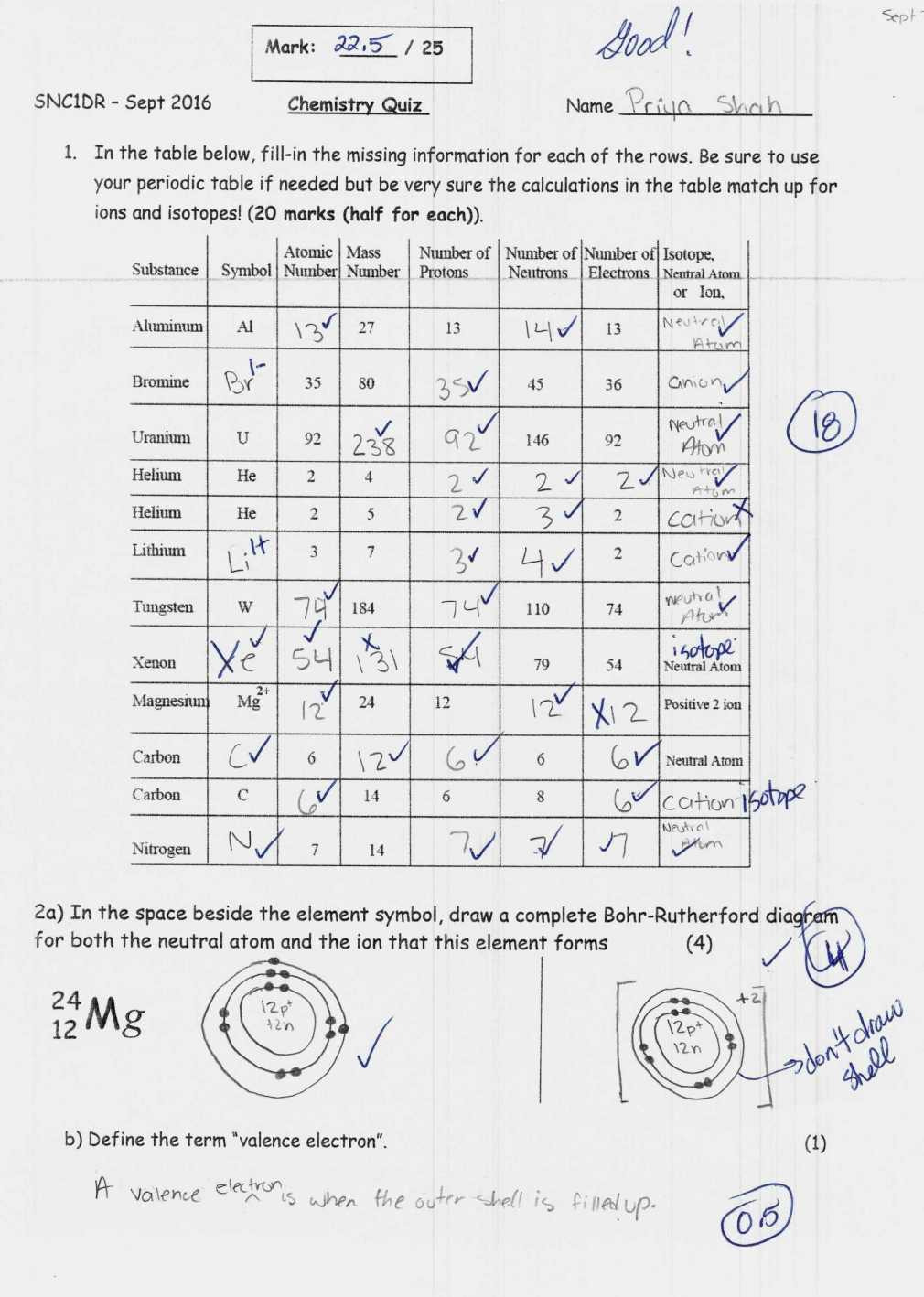
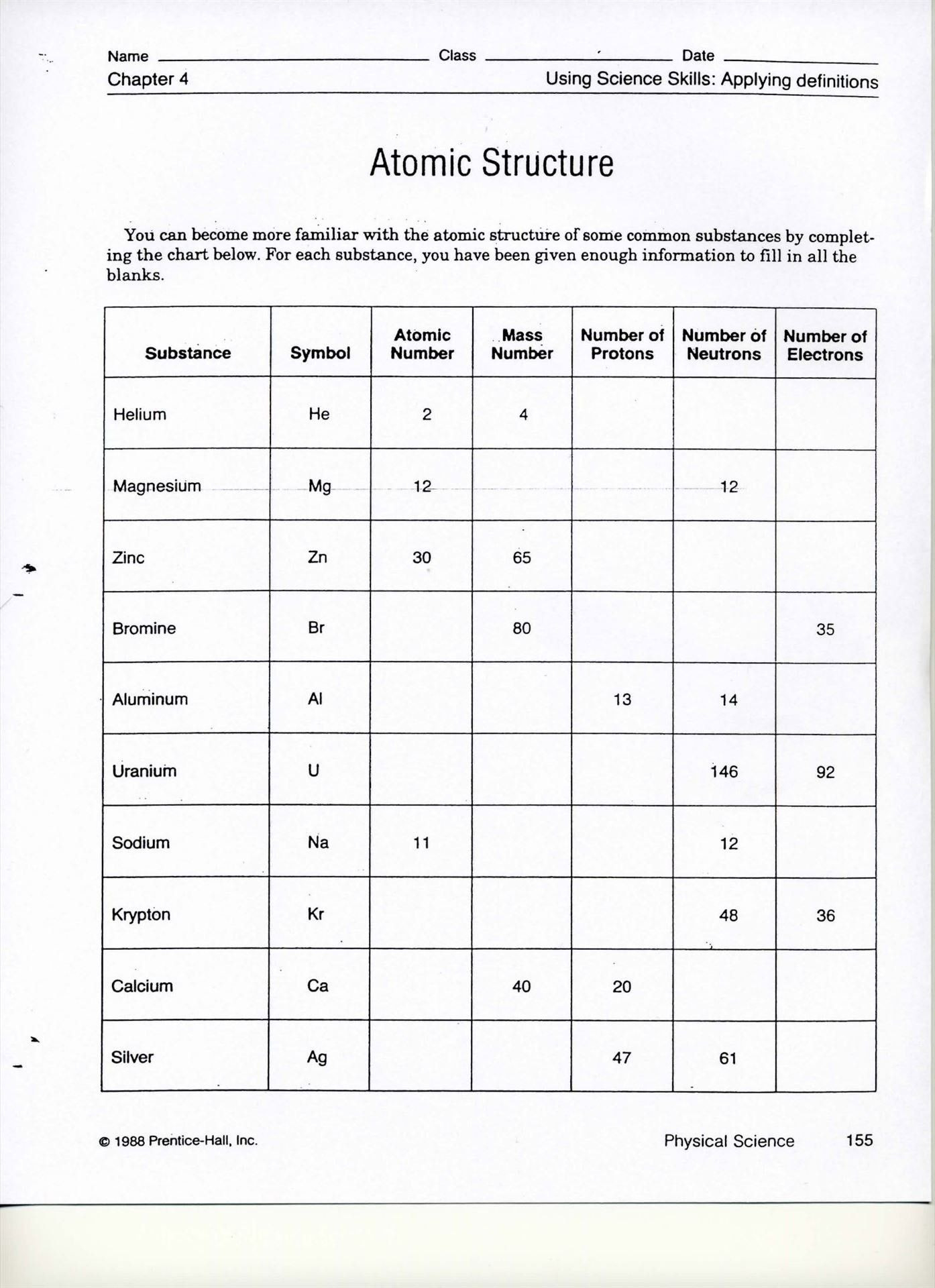
Atomic Structure Ions Isotopes Worksheet Answers Key

Understanding the complexities of atomic structure, ions, and isotopes forms a cornerstone in the study of chemistry. Whether you're a student looking to ace a chemistry exam, a teacher seeking to explain these concepts, or someone simply curious about the building blocks of matter, this comprehensive guide will walk you through the worksheet answers key for atomic structure, ions, and isotopes.
What is an Atom?
Atoms are the most basic unit of matter, comprised of:
- Protons - Positively charged particles found in the nucleus.
- Electrons - Negatively charged particles surrounding the nucleus in orbits or shells.
- Neutrons - Particles with no charge, found in the nucleus alongside protons.
Here’s a simple table illustrating an atom’s basic structure:
| Component | Charge | Location |
|---|---|---|
| Proton | +1 | Nucleus |
| Electron | -1 | Electron Cloud/Shells |
| Neutron | 0 | Nucleus |

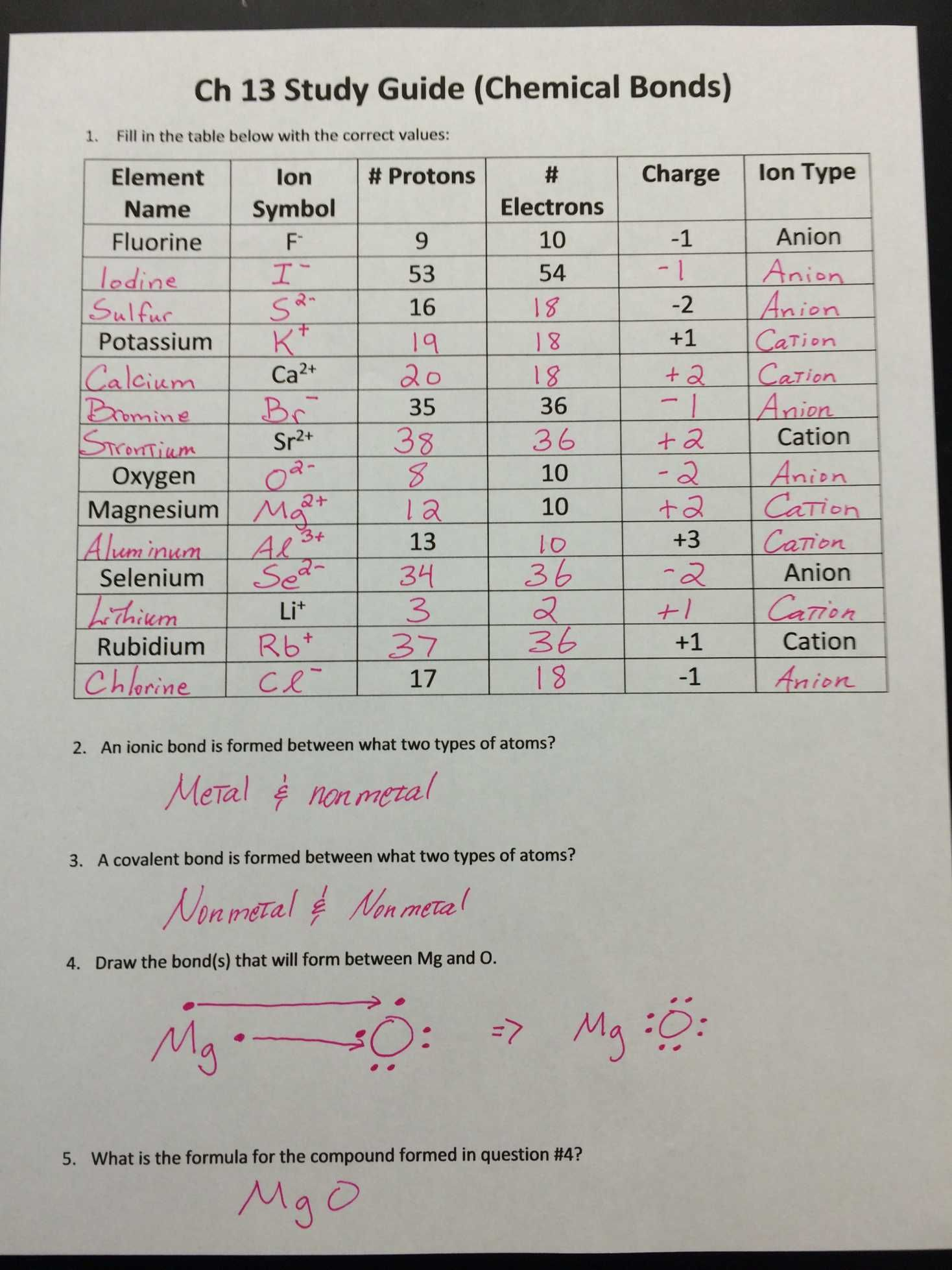
Ions: Electrically Charged Atoms

An ion is an atom or molecule that has gained or lost one or more electrons, resulting in a net positive or negative electric charge. There are:
- Cations - Positively charged ions formed when atoms lose electrons.
- Anions - Negatively charged ions formed when atoms gain electrons.
Here are some examples:
- Sodium (Na) loses one electron to become a sodium ion (Na+).
- Chlorine (Cl) gains one electron to become a chloride ion (Cl-).
⚠️ Note: Remember, an atom’s identity does not change when it becomes an ion; only its charge does.
Isotopes: Variations in an Element

Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. This difference affects the mass number but not the chemical properties of the element. For example:
- Carbon-12 has 6 protons and 6 neutrons.
- Carbon-14 has 6 protons and 8 neutrons.
Isotopes can be:
- Stable - Do not decay naturally.
- Unstable (Radioactive) - Undergo radioactive decay, emitting particles until they reach stability.
Worksheet Answer Key

Below is a sample of how a worksheet might present questions on these topics and the corresponding answers:
1. Atomic Structure

Question: How many protons, electrons, and neutrons are in a neutral atom of boron-11?
Answer: Boron-11 (B-11) has 5 protons, 5 electrons, and 6 neutrons. (5 protons come from the atomic number of boron; the 6 neutrons are derived from the mass number minus the number of protons).
2. Ions

Question: If a sulfur atom (S) gains 2 electrons, what ion is formed?
Answer: A sulfur atom with an extra 2 electrons will form a sulfide ion (S2-).
3. Isotopes

Question: How many neutrons are in an isotope of helium with a mass number of 4?
Answer: Helium-4 (He-4) has 2 protons and 2 neutrons.
Importance of Understanding Atomic Structure, Ions, and Isotopes
Having a robust grasp of these concepts:
- Helps in understanding chemical reactions where ions play crucial roles.
- Allows for better comprehension of radioactivity and nuclear chemistry through isotopes.
- Is essential in fields like radiology, medicine, and environmental science.
- Provides the foundation for more advanced topics like molecular structure and chemical bonding.
In summary, atoms are the foundational units of matter, ions are atoms with a charge due to electron loss or gain, and isotopes vary in neutron count but not in their atomic identity. These elements of chemistry are not only vital for academic success but also for understanding the world around us.
What’s the difference between an atom and an ion?

+
An atom is a neutral unit of matter that can have protons, neutrons, and electrons. An ion, on the other hand, is an atom or molecule with an unequal number of protons and electrons, making it electrically charged.
Can all elements have isotopes?
+
Yes, every element can have isotopes. Isotopes are variations of an element with different numbers of neutrons, but the same number of protons.
Why do we care about isotopes in chemistry?

+
Isotopes are significant in chemistry because they can have different properties, especially in nuclear reactions and medical applications like radiology.
What causes an atom to become an ion?

+
An atom becomes an ion by either gaining or losing electrons. This change in electron count affects the atom’s charge, creating an ion.