5 Essential Facts about Ions and Isotopes in Atomic Structure

Understanding the basic components of an atom is crucial for diving deeper into atomic structure. Today, we'll explore the fascinating world of ions and isotopes, which are pivotal in various scientific fields, including chemistry, physics, and nuclear science. By the end of this comprehensive exploration, you'll grasp how these particles define elements and influence the universe around us.
The Atom's Building Blocks

To start our journey, let's outline the fundamental particles that make up an atom:
- Protons: Positively charged particles located in the nucleus.
- Electrons: Negatively charged particles that orbit the nucleus.
- Neutrons: Neutral particles also located in the nucleus.
These particles define the atomic number, mass number, and other characteristics of an atom. Now, let's delve into how ions and isotopes differ from the typical atomic structure.
What Are Ions?

Ions are atoms or molecules that have lost or gained electrons, resulting in a net electrical charge. Here’s how they form:
- Cation Formation: When an atom loses electrons, it becomes positively charged. For example, sodium (Na) becomes Na+ when it loses one electron.
- Anion Formation: When an atom gains electrons, it gains a negative charge. For instance, chlorine (Cl) becomes Cl- when it gains one electron.
Ions are vital in:
- Chemical Bonds: Many ionic compounds form due to the attraction between positively and negatively charged ions.
- Electrical Conductivity: Ions in a solution or molten state conduct electricity.
Key Properties of Ions

| Type of Ion | Charge | Formation | Example |
|---|---|---|---|
| Cation | Positive | Loss of electrons | Na+ |
| Anion | Negative | Gain of electrons | Cl- |

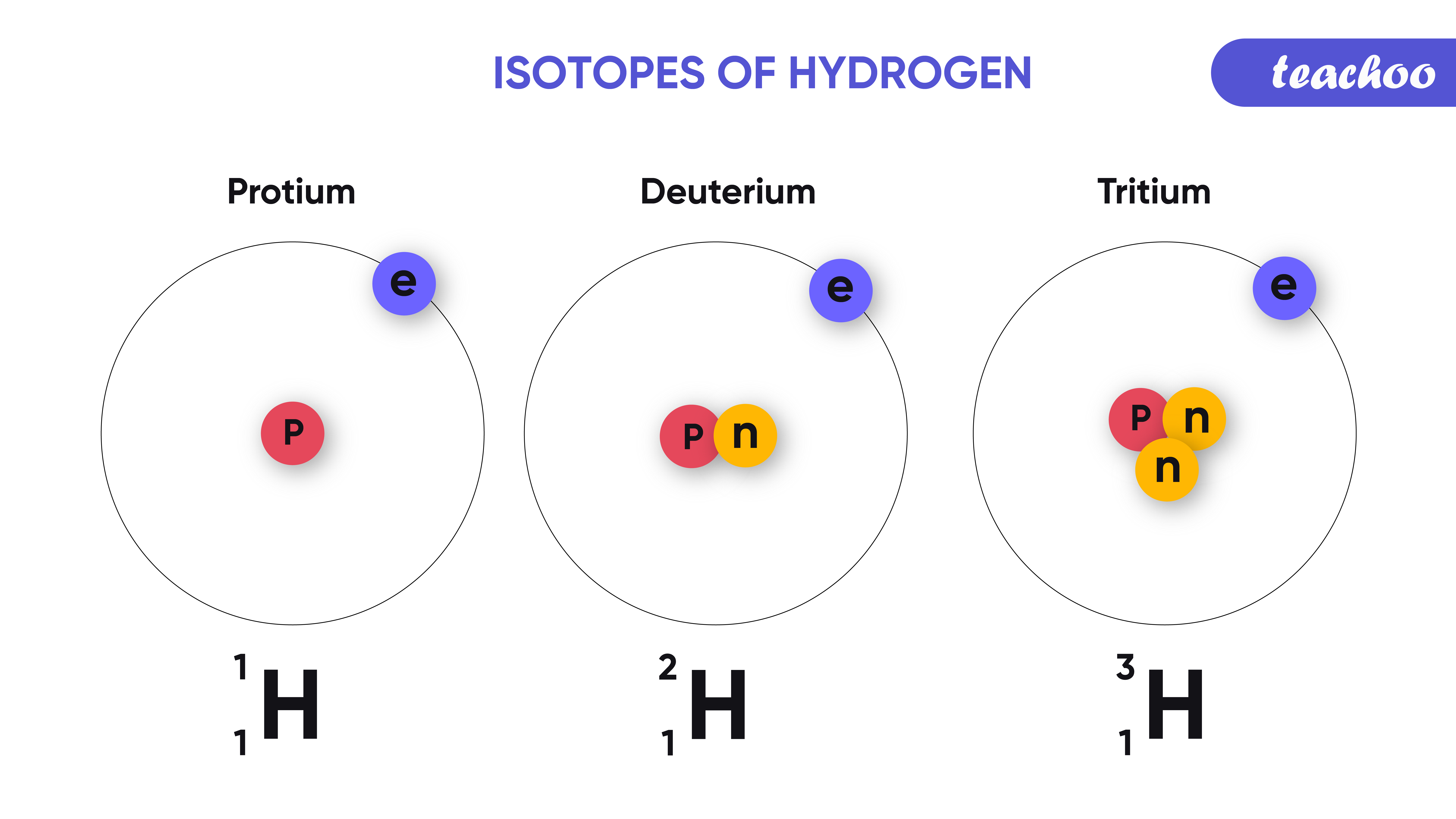
What Are Isotopes?

Isotopes are variants of an element that differ in the number of neutrons, while the number of protons remains unchanged. This difference leads to variations in mass and stability:
- Atomic Mass: Isotopes have different mass numbers due to varying neutron counts.
- Radioactivity: Some isotopes are unstable and radioactive, decaying over time to achieve stability.
Let’s look at some common isotopes:
- Carbon-12 (12C) and Carbon-14 (14C): Carbon-14 is used in radiocarbon dating.
- Uranium-235 (235U) and Uranium-238 (238U): Uranium-235 is used in nuclear reactors.
Properties of Isotopes

| Isotope | Protons | Neutrons | Mass Number | Stability |
|---|---|---|---|---|
| Carbon-12 | 6 | 6 | 12 | Stable |
| Carbon-14 | 6 | 8 | 14 | Radioactive |
| Uranium-235 | 92 | 143 | 235 | Fissile |
| Uranium-238 | 92 | 146 | 238 | Non-fissile |
Ions and isotopes play a crucial role in both natural phenomena and technological advancements. For instance, understanding isotopes like carbon-14 has allowed for breakthroughs in archaeology and dating ancient artifacts, while isotopes such as uranium are fundamental in energy production and medicine. Ions, with their electric charge, facilitate various chemical reactions essential for life and industry.
⚛️ Note: When discussing the stability of isotopes, it's worth mentioning that half-life is a key concept. The half-life is the time it takes for half of the radioactive isotope to decay, which can range from fractions of a second to billions of years depending on the isotope.
Final Thoughts
As we've explored, ions and isotopes are not just abstract concepts in atomic theory; they are the building blocks of our understanding of chemistry, physics, and life itself. Ions are involved in everything from the functioning of our nervous system to the electrical conductivity in our devices, while isotopes give us insights into the age of the Earth and tools for medical diagnostics and treatment.
This foundational knowledge helps us appreciate the complexity and interconnectedness of the atomic world. Each atom, with its potential to become an ion or variant isotope, contributes to the larger tapestry of matter that forms our universe. We've seen how the manipulation and understanding of these particles can lead to technological breakthroughs, environmental understanding, and medical advancements. By grasping these essential facts about ions and isotopes, we can better comprehend the dynamic nature of atomic structure and its applications.
What is the difference between an ion and an isotope?

+
An ion is an atom or molecule with a net electrical charge due to the gain or loss of electrons. An isotope, however, is a variant of an element that has the same number of protons but a different number of neutrons, leading to different mass numbers.
Why are isotopes important in nuclear energy?
+
Isotopes like Uranium-235 are used in nuclear reactors because they can undergo fission when hit by neutrons. This reaction releases energy, providing a source for electricity generation.
Can isotopes change into ions?

+
Yes, isotopes can change into ions if they either gain or lose electrons. The isotopic identity remains, but their ionic form will carry a charge. For example, a sodium-23 isotope can lose an electron to become a sodium-23 cation, Na+.