Bohr Model Mastery: Answers for Atomic Structure Worksheet

Mastering the Bohr Model: A Comprehensive Guide

The Bohr model, proposed by Niels Bohr in 1913, is a pivotal concept in understanding atomic structure. This model provides a simplified yet insightful way of visualizing the electron arrangement in an atom, offering both educators and students a useful tool for understanding the basics of atomic theory. In this blog post, we'll delve into the intricacies of the Bohr model, its significance, and how to excel in answering related questions in atomic structure worksheets.
Understanding the Bohr Model

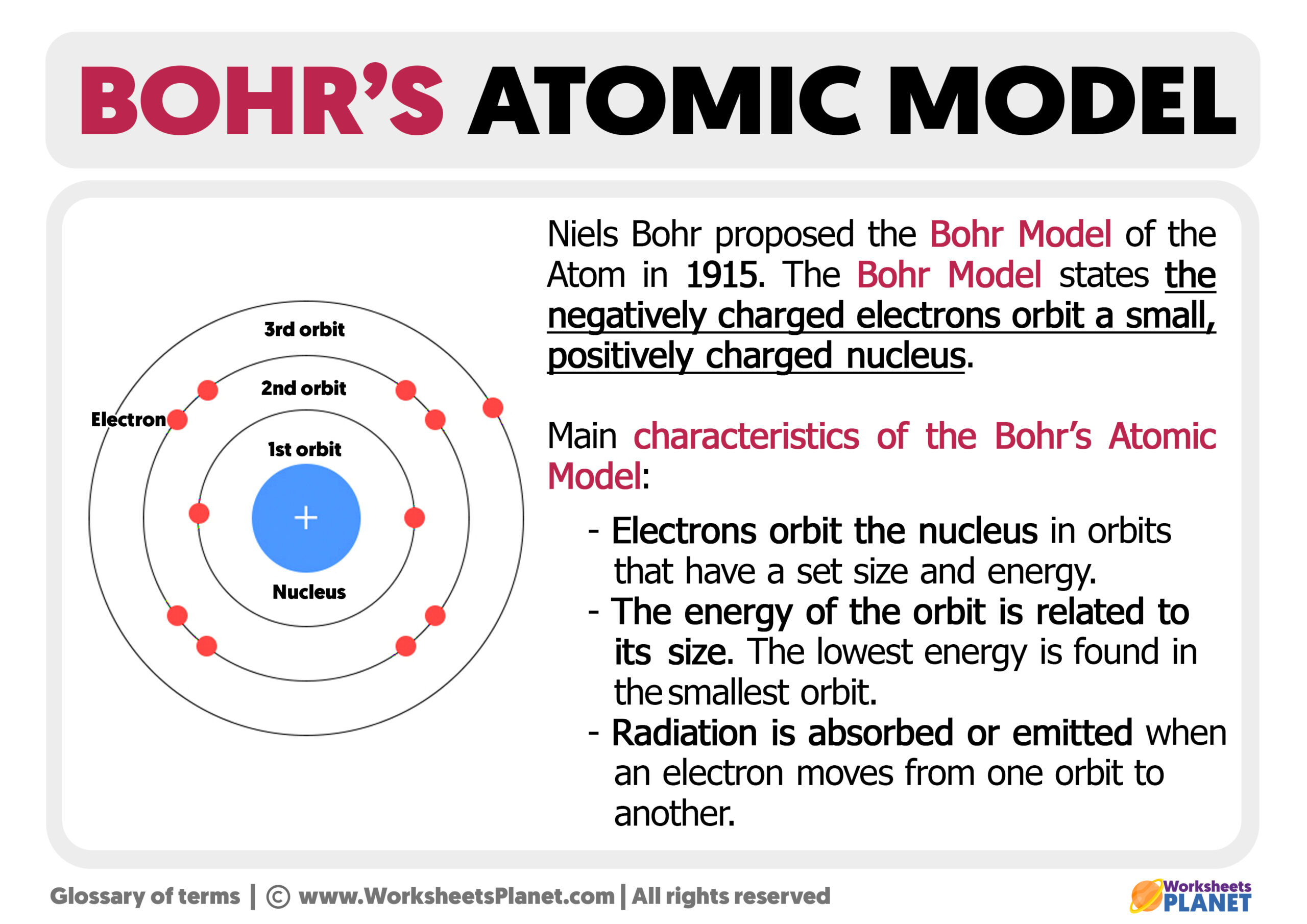
Niels Bohr introduced his model by expanding upon the earlier atomic theories, especially those of J.J. Thomson and Ernest Rutherford. Here's a brief overview of what the Bohr model entails:
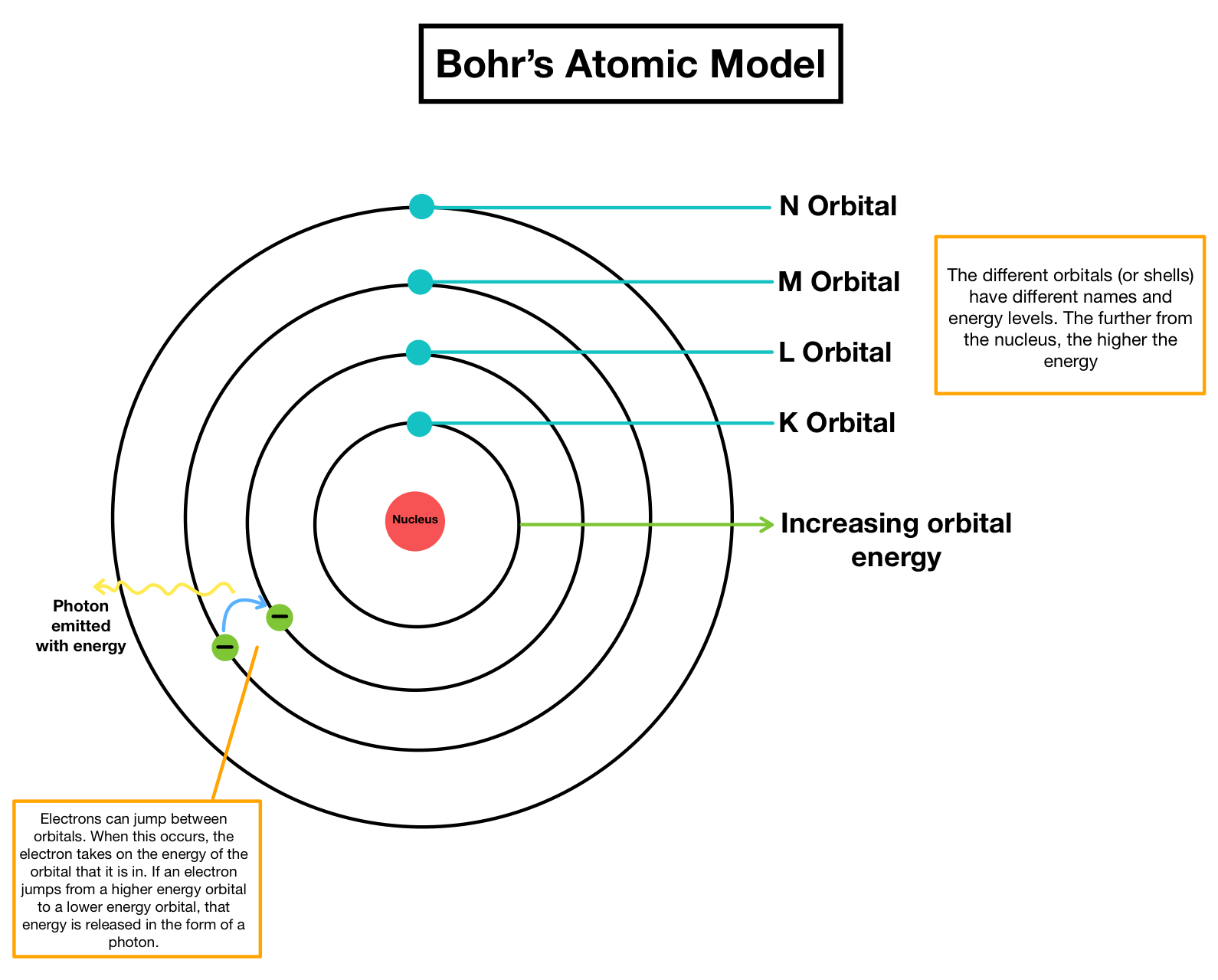
- Electron Orbits: Electrons orbit the nucleus at fixed distances known as energy levels or electron shells.
- Quantum Numbers: Each shell corresponds to a specific quantum number, with the closest shells having the lowest energy states.
- Energy Transitions: When electrons jump between these energy levels, they absorb or emit energy in the form of photons.
Fundamental Principles of the Bohr Model

The Bohr model is not just a visual aid; it's grounded in several fundamental principles:
1. Stationary States:

Electrons in an atom can only exist in certain fixed energy states. These states are referred to as stationary states or orbits.
2. Quantization of Energy:

Energy can be absorbed or emitted by the electron only in discrete quantities or quanta. This leads to the concept of energy levels where each level represents a different amount of energy.
3. Electromagnetic Radiation:

When an electron moves from a higher to a lower energy level, the energy difference is released as electromagnetic radiation. Conversely, absorbing energy can cause an electron to jump to a higher level.
4. Angular Momentum:

Electrons in the Bohr model have quantized angular momentum, which can only be a multiple of Planck's constant divided by 2π.
Applying the Bohr Model in Atomic Structure Worksheets
Here are steps and strategies to master questions related to the Bohr model in atomic structure worksheets:
Identify the Atom:

- Recognize the element from the given atomic number or periodic table.
- Determine the number of protons, neutrons, and electrons from the element's details.
Shell Filling:

- The first shell (K shell) holds 2 electrons.
- The second shell (L shell) can hold up to 8 electrons.
- Continue filling subsequent shells with 8, 18, or 32 electrons based on the periodic table's electron configuration trend.
Draw the Bohr Diagram:
- Draw the nucleus in the center, indicating protons and neutrons.
- Draw circles around the nucleus for each shell.
- Place electrons in their respective shells using dots or X's to represent them.
💡 Note: In atomic structure questions, electrons usually fill the shells starting from the innermost shell and follow the order of increasing energy levels.
Common Questions in Atomic Structure Worksheets

How Many Shells Do I Draw?

- Draw shells until all electrons are accounted for. Remember, electrons occupy the lowest possible energy levels first.
Electron Transition and Energy:

- Calculate the energy difference when electrons move between shells using the formula ΔE = E(final) - E(initial).
Valence Electrons:

- Identify the outermost shell to determine the valence electrons, which are crucial for understanding chemical behavior.
Conclusion

By understanding the Bohr model and its application to atomic structure, you can navigate through worksheet questions with greater ease. This model simplifies the complex interactions within atoms, providing a foundation for further quantum mechanical principles. It's worth noting that while the Bohr model offers simplicity, it has limitations when dealing with more complex atoms or phenomena like electron spin, which are better explained by quantum mechanics. However, for introductory chemistry and basic atomic structure, it remains an invaluable educational tool.
How accurate is the Bohr model in representing real atoms?
+The Bohr model is a simplified representation of atomic structure, providing a foundational understanding of electron placement and energy levels. However, it doesn’t account for nuances like electron spin or quantum superposition, which are crucial in modern quantum mechanics. It’s accurate for basic single-electron systems like hydrogen but becomes less precise for multi-electron atoms.
Why do electrons orbit the nucleus without losing energy?
+In classical physics, electrons should lose energy and spiral into the nucleus due to electromagnetic radiation. Bohr’s model introduced the concept of quantized energy levels where electrons only lose or gain energy when transitioning between these levels, thereby not continuously losing energy in the process.
Can the Bohr model predict chemical reactivity?
+Yes, to some extent. The Bohr model allows you to determine the valence electrons (electrons in the outermost shell), which largely dictates an element’s reactivity and bonding behavior. However, for more accurate predictions, one needs to consider quantum mechanics and molecular orbital theory.
How does the Bohr model differ from the modern quantum mechanical model of the atom?
+The Bohr model suggests electrons move in fixed circular orbits, while quantum mechanics describes electrons as probability clouds or orbitals, indicating the likelihood of finding an electron in various regions around the nucleus. This accounts for electron spin, quantum numbers, and the probabilistic nature of electron location.
Is the Bohr model still used in chemistry education?
+Yes, the Bohr model is still used due to its simplicity and ease of understanding basic atomic principles. It’s often the first model taught in chemistry courses to provide an intuitive understanding of electron configuration and atomic structure, though it’s supplemented with more accurate models as students advance in their studies.