7 Key Insights from Atomic Basics Worksheet Answers
The realm of chemistry, particularly the study of atoms, is foundational for understanding the physical and chemical properties of matter. Whether you're a student delving into the basics of atomic structure for the first time or someone looking to brush up on fundamental concepts, Atomic Basics Worksheet Answers can provide key insights into the building blocks of our universe. Here, we explore seven essential learnings from such worksheets that can pave the way for a deeper comprehension of chemistry.
The Atomic Model: A Historical Perspective

- Early Concepts: Initially, ancient Greek philosophers like Democritus proposed that all matter consisted of 'atomos' (indivisible particles), though this was purely speculative.
- John Dalton's Model: In the early 19th century, Dalton introduced the idea of atoms as indivisible spheres with different weights, laying the groundwork for modern atomic theory.
- Thomson's Plum Pudding Model: J.J. Thomson discovered the electron, leading to the 'plum pudding' model where electrons were embedded in a positively charged material.
- Rutherford's Gold Foil Experiment: This experiment demonstrated that atoms have a small, dense nucleus, overturning previous models.
- Bohr's Model: Niels Bohr proposed that electrons move in fixed orbits around the nucleus, explaining atomic spectra.
- Quantum Mechanical Model: Modern understanding involves quantum mechanics, where electron locations are described by probability clouds rather than fixed orbits.
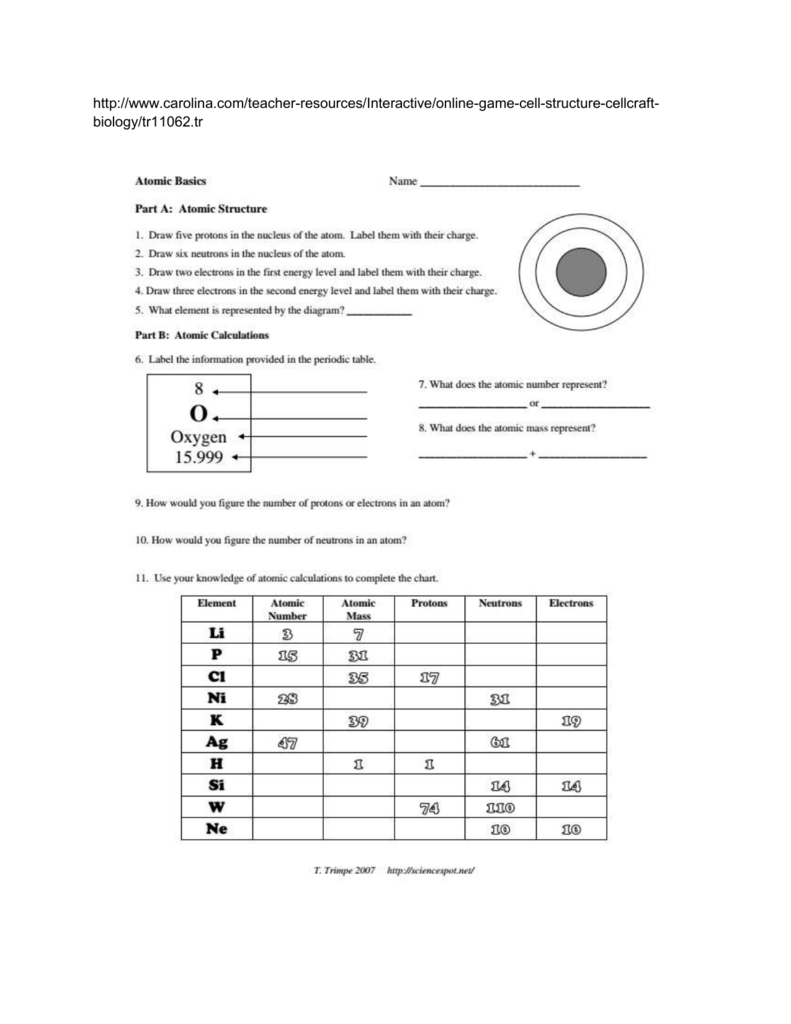
Components of the Atom

An atom consists of three primary types of particles:
- Protons: Positively charged particles found in the nucleus, which determine an element's atomic number.
- Neutrons: Neutral particles in the nucleus that contribute to the mass of the atom without affecting its charge.
- Electrons: Negatively charged particles orbiting the nucleus, which play a crucial role in chemical reactions and bonding.
Atomic Number and Mass Number

- The atomic number is the number of protons in an atom's nucleus. It uniquely identifies an element.
- The mass number is the sum of protons and neutrons in the nucleus, representing the approximate mass of an atom.
📝 Note: Isotopes of an element have the same atomic number but different mass numbers due to variations in neutron count.
Isotopes and Atomic Mass

| Isotope | Mass Number | Neutrons |
|---|---|---|
| Carbon-12 | 12 | 6 |
| Carbon-13 | 13 | 7 |
| Carbon-14 | 14 | 8 |

Isotopes are variants of an element with differing numbers of neutrons. Understanding this concept helps explain atomic mass:
- The average atomic mass on the periodic table is a weighted average of all naturally occurring isotopes of an element.
Electron Configuration

How electrons are arranged in an atom is critical for understanding its behavior:
- Energy Levels: Electrons occupy different energy levels or shells, with lower energy levels closest to the nucleus.
- Subshells: Within these levels, electrons fill subshells (s, p, d, f), which further divide into orbitals.
- Aufbau Principle: Electrons fill lower energy orbitals first.
- Hund's Rule: When filling degenerate orbitals (those with the same energy), electrons will first occupy each with one electron before pairing.
- Pauli Exclusion Principle: No two electrons in an atom can have the same set of four quantum numbers.
🔬 Note: Electron configurations can help predict an element's reactivity and chemical properties.
Valence Electrons and Chemical Bonding

- Valence Electrons: The outermost shell's electrons are involved in chemical bonding.
- Atoms with a full valence shell tend to be chemically stable; otherwise, they bond to achieve this stability.
- Chemical bonds form through sharing or transferring electrons, creating compounds.
Periodic Trends

Periodic tables organize elements by increasing atomic number and reveal various trends:
- Atomic Radius: Increases down a group, decreases across a period due to nuclear charge.
- Ionization Energy: Energy needed to remove an electron from an atom; generally decreases down a group and increases across a period.
- Electronegativity: Tendency to attract a bonded pair of electrons; similar trends to ionization energy.
📊 Note: These trends help predict how elements will react with each other in chemical reactions.
To wrap up, understanding atomic basics through worksheets provides not just knowledge but a lens through which we can view the intricate dance of particles that forms the world around us. From the historical evolution of the atomic model to the practical applications of electron configurations and periodic trends, these insights serve as the foundation for grasping more complex chemical concepts. By understanding these fundamental principles, students and enthusiasts alike can better comprehend how the elements interact to shape matter, energy, and life itself.
What is the significance of the atomic number?
+
The atomic number defines an element by determining the number of protons, which also gives an indication of the electron number when the atom is in its neutral state.
How can understanding isotopes be useful?

+
Knowledge of isotopes is crucial in various fields like archaeology for dating, in medicine for treatments like radiation therapy, and in industry for tracing processes.
Why is electron configuration important?

+
Electron configuration is key to understanding an element’s chemical behavior, how it bonds, and its placement in the periodic table, impacting its reactivity and properties.