Mastering pH and pOH: Your Ultimate Guide

The world of chemistry is filled with various measurements and scales that help us understand the properties of substances, and among the most essential are pH and pOH. Both scales are pivotal in assessing the acidity or basicity of solutions, which can have profound implications in various scientific fields including environmental science, biology, and pharmaceuticals. In this guide, we'll delve deeply into mastering the nuances of pH and pOH, offering you a comprehensive understanding and practical applications.
Understanding pH and pOH

pH stands for “potential of Hydrogen,” indicating the negative logarithm (base 10) of the concentration of hydrogen ions in a solution. Conversely, pOH measures the concentration of hydroxide ions, calculated similarly as the negative logarithm of the hydroxide ion concentration. Here’s a quick breakdown:
- pH: -log[H+] where [H+] is the molar concentration of H+ ions
- pOH: -log[OH-] where [OH-] is the molar concentration of OH- ions
The pH and pOH scales range from 0 to 14, with pure water being neutral at pH 7 (pOH 7). Solutions with a pH below 7 are acidic, and those above 7 are basic or alkaline. Understanding this duality allows us to:
- Determine the nature of a solution
- Predict chemical behavior
- Calculate necessary titrations in laboratory conditions
💡 Note: The sum of pH and pOH at 25°C is always 14. If you know one, you can find the other by subtracting it from 14.
How to Measure pH and pOH

Measurement methods for pH and pOH vary from simple to advanced:
- Litmus Paper: A basic method offering a quick pH indication. Blue litmus turns red in acid, while red turns blue in base.
- pH Meter: This device gives precise pH readings by measuring the electrical potential difference between a pH-sensitive electrode and a reference electrode.
- Colorimetry: Using pH indicators (like phenolphthalein or methyl orange) which change color at different pH levels, this method can also be used to visually estimate pOH.
- Electrochemical Methods: For industrial applications, techniques like potentiometry provide real-time, continuous monitoring of pH or pOH.
When using pH meters, ensure they are properly calibrated, ideally with buffer solutions of known pH values. Calibrated meters provide accurate readings essential for quality control in fields like food processing, environmental monitoring, or drug formulation.
Converting pH to pOH and Vice Versa
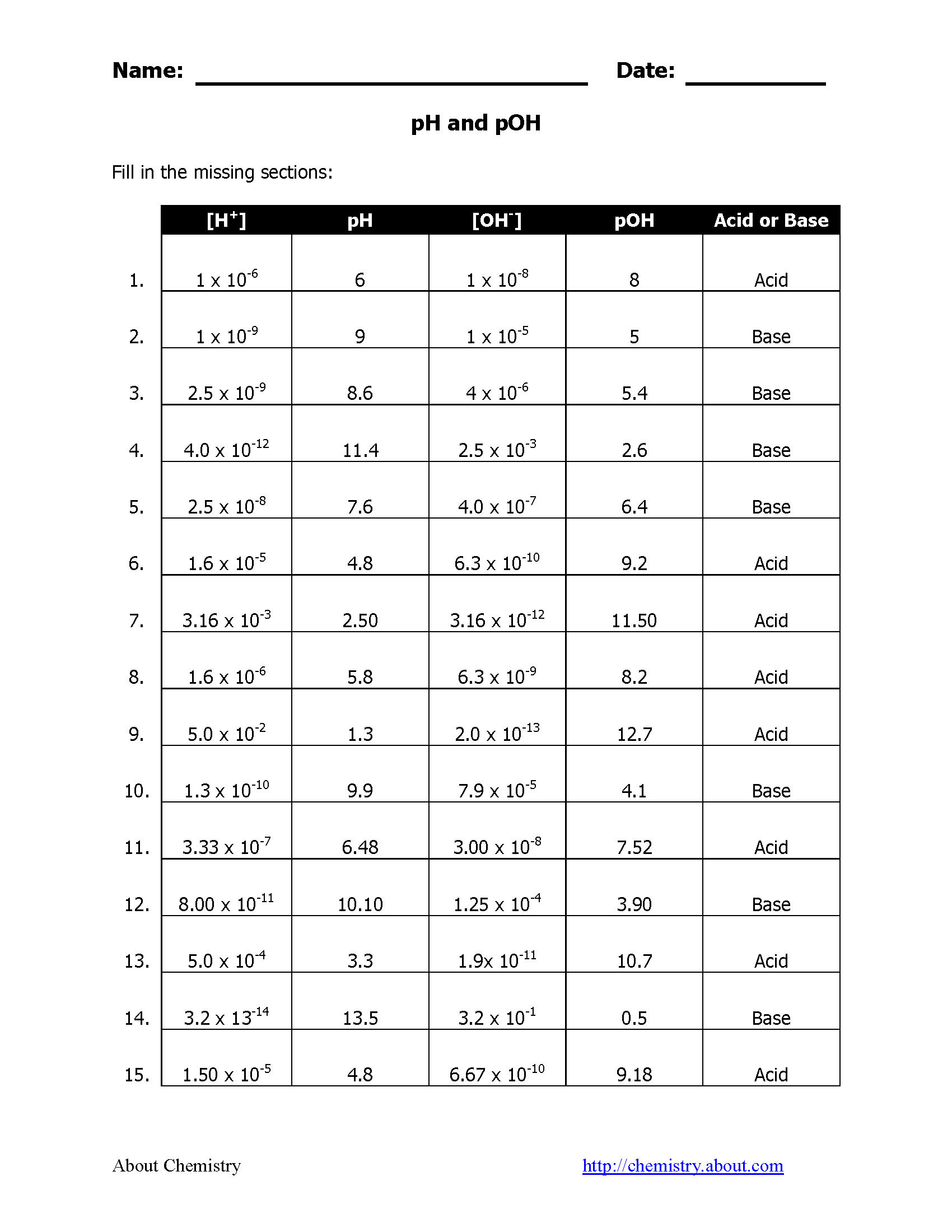
Understanding the interrelationship between pH and pOH is crucial for quick conversions:
- Given pH, you can find pOH by subtracting the pH value from 14. For example, if a solution has a pH of 8, then pOH would be 14 - 8 = 6.
- The reverse calculation also holds true; if pOH is known, subtract it from 14 to get pH.
Here's a handy table for quick reference:
| pH | pOH | Nature of Solution |
|---|---|---|
| 0 - 6 | 8 - 14 | Acidic |
| 7 | 7 | Neutral |
| 8 - 14 | 0 - 6 | Basic/Alkaline |

Practical Applications of pH and pOH

The measurement and control of pH and pOH have extensive applications:
- Water Treatment: Ensuring safe drinking water requires maintaining a specific pH range to prevent corrosion or contamination.
- Agriculture: Soil pH can significantly affect plant growth, nutrient availability, and microbial activity.
- Pharmacology: Drug stability, solubility, and absorption rates can be influenced by pH.
- Biological Processes: Enzymatic reactions in the body require specific pH levels for optimal functioning.
Moreover, industries like:
- Food manufacturing use pH to control fermentation, spoilage, and taste.
- Chemical engineering where pH adjustments are critical in processes like precipitation and crystallization.
The Importance of Calibration

Calibration of pH meters involves adjusting the meter with buffer solutions that have known pH values. This process ensures:
- Accurate readings by compensating for sensor wear and temperature variations.
- Traceability for laboratory standards and regulatory compliance.
🔎 Note: For calibration, use at least two buffer solutions with a pH difference of at least two units to ensure the linearity of the electrode.
Summary

Mastering pH and pOH provides an invaluable toolset for those involved in chemistry, biology, and related fields. By understanding these scales, we can manipulate chemical environments for beneficial outcomes, from improving plant growth in agriculture to optimizing drug efficacy in pharmaceuticals. This guide has explored the core principles, measurement techniques, and practical applications of pH and pOH, offering a foundational knowledge that bridges theory and practice.
What does pH stand for?

+
pH stands for “potential of Hydrogen,” a measure of the concentration of hydrogen ions in a solution.
How does temperature affect pH readings?

+
Temperature can alter the ionization of water, causing shifts in pH. Typically, pH decreases as temperature increases due to the enhanced dissociation of water into hydrogen and hydroxide ions.
Why is pH important in biological systems?

+
pH influences enzyme activity, cell membrane stability, and the overall function of biological systems. Many life processes are pH-sensitive, requiring homeostasis for optimal health.
Can I measure pOH directly?

+
While most instruments measure pH, pOH can be calculated indirectly by subtracting pH from 14. There are few direct methods for pOH measurement as it correlates with pH.
What are some common pH indicators used in labs?

+
Common pH indicators include:
- Phenolphthalein - changes from colorless to pink at a pH above 8.2
- Litmus - turns blue in bases and red in acids
- Methyl orange - changes from red in acids to yellow in bases
- Universal indicator - provides a broad color change across the pH range