5 Essential Tips for Mastering Acids/Bases & pH Worksheet Answers

Acid/base chemistry can seem challenging at first, but with the right approach and understanding, it can be an engaging and manageable part of your chemistry curriculum. Whether you're a high school student grappling with the basics or a seasoned chemist exploring more intricate reactions, understanding pH and how acids and bases interact is essential. Here are five critical tips to master your acids/bases and pH worksheet answers, enhancing your problem-solving skills and conceptual understanding.
1. Understand the Basics of pH

The pH scale is a measure of how acidic or basic a solution is. Here are some key points:
- The pH scale ranges from 0 to 14, where 0 is extremely acidic, 7 is neutral, and 14 is extremely basic.
- A pH value of 7 indicates that the concentration of H+ ions equals that of OH- ions.
- The pH of a solution can be calculated using the equation pH = -log[H+].
📌 Note: Remember that pH is logarithmic; thus, a change of one pH unit represents a tenfold change in H+ concentration.
2. Master the Key Concepts

Before you dive into complex problems, make sure you understand these foundational concepts:
- Strong vs. Weak Acids/Bases: Strong acids/bases dissociate completely in water, while weak acids/bases partially dissociate.
- Acid-base Conjugates: In an acid-base reaction, an acid turns into its conjugate base, and a base into its conjugate acid.
- Ionization and Dissociation: Understand how different compounds behave in water.
📌 Note: Practice identifying strong and weak acids/bases using their formulas, not just memorizing lists.
3. Use the Ka and Kb Constants

The acid dissociation constant (Ka) and the base dissociation constant (Kb) are crucial for understanding the strength of acids and bases. Here's how to work with them:
- Ka = [H+][A-] / [HA] for acids and Kb = [OH-][B+] / [BOH] for bases.
- Remember, a larger Ka or Kb indicates a stronger acid or base, respectively.
- They are related by the equation Ka x Kb = Kw, where Kw is the ion-product constant of water at a given temperature, usually 1 x 10-14 at 25°C.
📌 Note: While solving for pH or pOH, you might need to use approximations or the quadratic formula, especially with weak acids/bases.
4. Practice Balancing Equations

Balancing chemical equations is fundamental:
- Start by identifying the reactants and products.
- Balance the atoms on both sides of the equation using coefficients.
- For acid-base reactions, consider the neutralization reaction, which can be simplified as Acid + Base → Salt + Water.
Here's an example:
| Reactant | + | Reactant | → | Product | + | Product |
|---|---|---|---|---|---|---|
| HCl | + | NaOH | → | NaCl | + | H2O |

📌 Note: Ensure you balance for each element and account for the charges when dealing with polyatomic ions.
5. Solve Problems Systematically

Here's a step-by-step approach to problem-solving in acid/base chemistry:
- Identify the Type of Problem: Is it titration, pH calculation, or solubility?
- Select the Correct Equation or Formula: Use Ka, Kb, Henderson-Hasselbalch, or other relevant formulas.
- Set Up Your Calculation: Make sure you understand all variables and constants involved.
- Calculate Step by Step: Follow the logic, ensuring your math is correct.
- Check Units and Dimensions: Your answer must reflect the correct units.
In summary, mastering acids/bases and pH worksheet answers isn't just about memorizing formulas; it's about understanding the underlying principles, knowing when to apply which concept, and being meticulous in your calculations. By following these tips, you'll find yourself navigating through complex problems with more ease and confidence, whether you're calculating pH, dealing with buffers, or determining the solubility of salts in aqueous solutions.
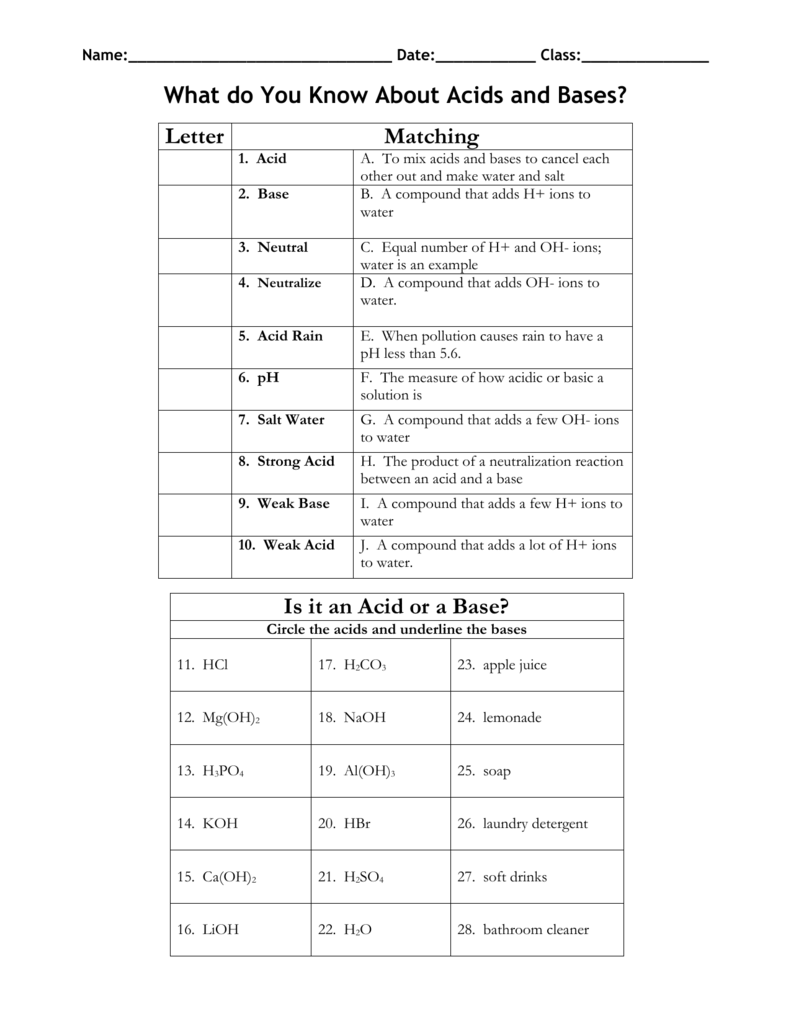
What’s the difference between an acid and a base?
+
Acids are substances that donate protons (H+ ions) in solution, while bases accept protons or provide OH- ions. Acids generally have a pH less than 7, while bases have a pH greater than 7.
How do you calculate pH?

+
pH is calculated using the formula pH = -log[H+], where [H+] is the concentration of hydrogen ions in moles per liter.
Why is pH important in real life?

+
pH affects the environment, biology, and industry. For example, it influences the growth of plants, the functioning of enzymes in our body, and the corrosion of metals. Proper pH levels are crucial for health, agriculture, and technology.