Acids and Bases Nomenclature Simplified: Interactive Worksheet

Welcome to this comprehensive guide on simplifying the nomenclature of acids and bases. Understanding how to name acids and bases correctly is crucial for students and professionals in the fields of chemistry and related sciences. In this interactive worksheet, we'll explore the basics of acid and base naming conventions, look at some common examples, and clarify any confusions through interactive elements that will make learning both fun and effective.
Basic Principles of Acid Nomenclature

Acids are known for their ability to donate protons (H+) or accept an electron pair in chemical reactions. Here’s how you name them:
- Binary acids: These acids consist of hydrogen and one other element. Naming them follows this pattern:
- Use the prefix hydro-
- Append the root of the second element.
- Add the suffix -ic, and then the word "acid".
Example: HCl is hydrochloric acid.
- Oxyacids: These contain hydrogen, oxygen, and another element. The naming follows these rules:
- If the anion ends in -ate, the acid name ends in -ic acid.
- If the anion ends in -ite, the acid name ends in -ous acid.
Example: H2SO4 (sulfate) is sulfuric acid, while H2SO3 (sulfite) is sulfurous acid.
Naming Bases
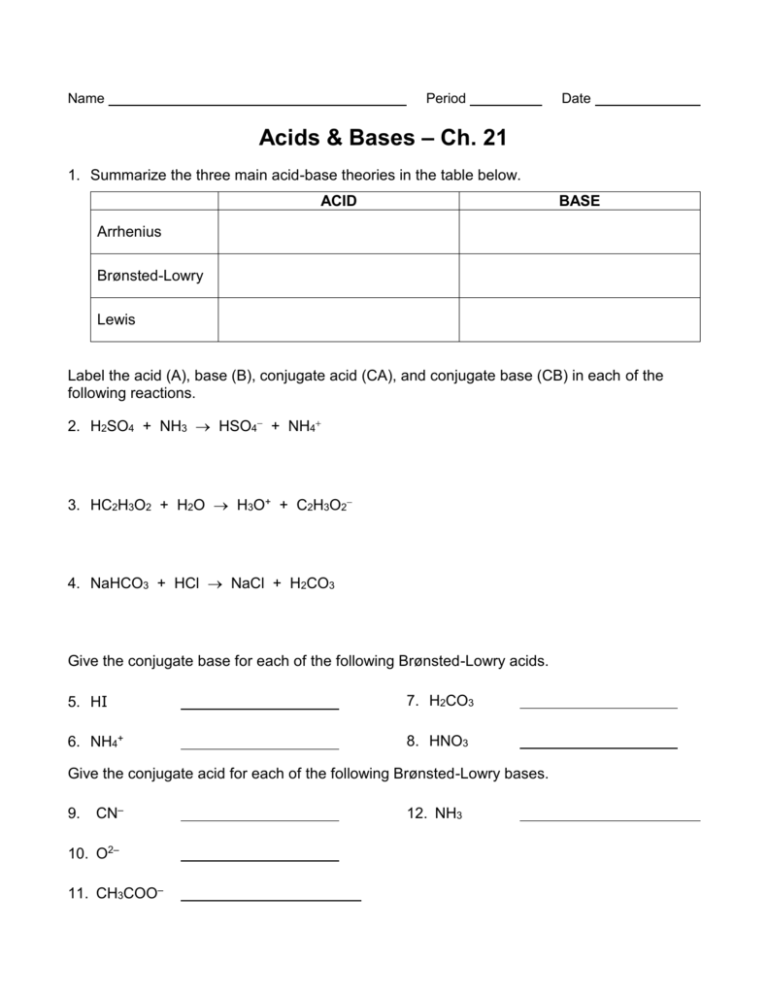
Bases are substances that can accept protons (H+) or donate an electron pair. Bases are typically named as follows:
- Metal Hydroxides: The name is composed of:
- The name of the metal
- The word "hydroxide"
Example: NaOH is sodium hydroxide.
- Ammonia and its derivatives: Ammonia itself (NH3) remains ammonia, but if it's protonated, it becomes ammonium hydroxide (NH4OH).
Interactive Nomenclature Worksheet
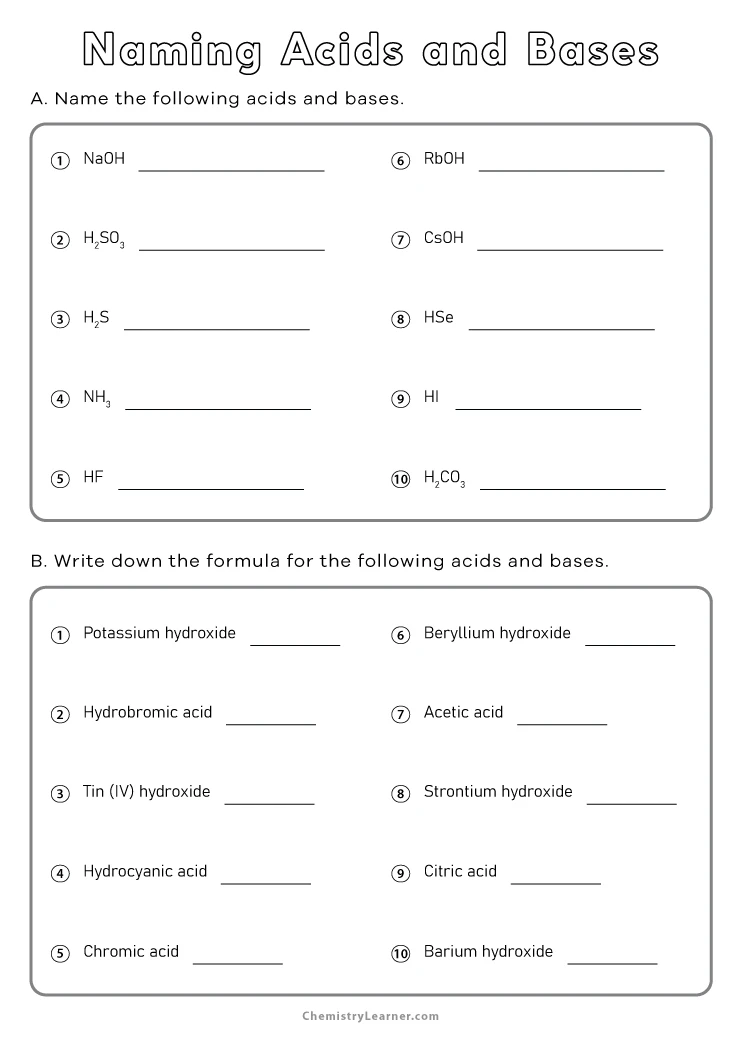
Here’s an interactive section to test your understanding:
| Chemical Formula | Name the Acid or Base |
|---|---|
| HClO | |
| Ca(OH)2 | |
| HNO2 | |
| Al(OH)3 |

💡 Note: Use the provided rules to name the acids and bases listed in the table above.
Advanced Nomenclature Tips

- Polyatomic Ions: Recognize common polyatomic ions and their acid derivatives. For instance, the phosphate ion (PO43-) leads to phosphoric acid (H3PO4).
- Prefixes and Suffixes: When dealing with different oxidation states, use Greek numerical prefixes (e.g., hypo-, per-) to differentiate between related acids.
- Order Matters: When writing the formula for an oxyacid, always place hydrogen atoms first, followed by the non-metal bonded to oxygen.
In this journey through acid and base nomenclature, we've covered the fundamental rules, interactive learning, and some advanced tips. By practicing with interactive worksheets and understanding the chemical properties, you're well on your way to mastering this essential aspect of chemistry. Let's keep in mind that learning these principles allows for better communication of chemical structures and reactions within the scientific community, facilitating research, education, and industrial applications.
What is the difference between an acid and a base?

+
An acid donates protons (H+) or accepts an electron pair, whereas a base accepts protons or donates an electron pair. This is according to the Bronsted-Lowry definition.
Why do acids have “hydro-” in their names?

+
The “hydro-” prefix is used for binary acids, which only contain hydrogen and one other element. It indicates the presence of hydrogen in the compound.
How do you name acids that come from polyatomic ions?

+
Polyatomic ions in acids follow the -ic and -ous naming conventions based on the anion endings:
- -ate ions become -ic acid
- -ite ions become -ous acid
Can a compound be both an acid and a base?

+
Yes, some substances can act as both an acid and a base depending on the chemical environment. These are called amphiprotic (or amphoteric) compounds. Water (H2O) is an example that can either donate or accept a proton.
What is the importance of understanding acid-base nomenclature?

+
Understanding acid-base nomenclature is crucial for:
- Communicating chemical reactions effectively.
- Identifying the properties and behavior of substances in various chemical contexts.
- Ensuring safety and proper handling in industrial and laboratory settings.
- Advancing chemical research and education.